Biomedical Engineering Reference
In-Depth Information
-
O
O
-
2-
N
+
O
O
NO
2
O
O
O
O
N
N
O
O
O
O
O
O
2e
-
O
O
O
O
O
O
N
N
C
1
O
4e
-
O
O
O
N
+
O
O
NO
2
-
O
-
2M
2M
2+
2M
2+
-
O
O
-
C
6
4+
2+
N
+
O
NO
2
O
O
O
O
O
M
2+
M
2+
N
N
O
O
O
O
O
O
2e
-
O
O
O
O
O
O
N
N
M
2+
M
2+
C
5
O
O
O
O
N
+
O
O
NO
2
-
O
-
Scheme 5 Complexation of the planar reduction product of ligand 17 with Zn
2+
and Cd
2+
with a
LM
2
stoichiometry
prepared and studied [
33
,
34
]. The reactivity of compound 9 to anions was also
studied for comparison purposes.
NO
2
R
O
O
O
O
H
H
O
O
O
N
N
N
N
N
H
O
O
N
O
H
O
O
O
O
18
R=NO
2
20
R=N(CH
3
)
2
NO
2
R
19
Anion complexation experiments were carried out by adding 10 equiv. of the
corresponding anion (F
,Cl
,Br
,I
,H
2
PO
4
), as tetrabutylammonium (TBA)
salt, to the solutions (10
2
M) of the different receptors in acetonitrile.
The most remarkable effect was the selective color noted with 18 and 19 upon
the addition of fluoride, either alone or in competitive assays (Fig.
19
). The NMR
studies of the complexation process reveal that two consecutive reactions take place
in the presence of fluoride. Thus, after adding 1 equiv. of fluoride, a complex with a
1:1 stoichiometry and the geometry shown in Fig.
20
(left) formed.
However, color development was due to the deprotonation reaction taking place
in the presence of at least 2 equiv. of fluoride. The color exhibited by ligands 18 and
19 upon the addition of fluoride ion is probably due to deprotonation, which leads to
a charge-transfer complex [Fig.
20
(right)]. This suggestion agrees not only with the
color extinction which occurs in the presence of water, but also with the results
obtained in the additional experiments carried out with tetrabutylammonium
t
-butoxide [TBA(
t
-BO)] and tetrabutylammonium hydroxide (TBAOH). Finally,
this proposal is reinforced by the behavior of ligand 20; its substitution, with





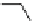

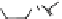
















































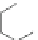





































































































































































Search WWH ::

Custom Search