Biomedical Engineering Reference
In-Depth Information
N
R
R
R
N
N
N
N
N
N
O
N
N
N
O
O
O
O
O
O
O
N
N
N
N
N
N
N
R
R
R
H
(a)
(b)
(c)
(d)
Fig. 11 Possible complexing arrangements suggested to account for the fluorescence results
obtained for the different ligands and cations
1,2
1,0
Zn2+
Cu2+
Ni2+
Pb2+
0,8
0,6
0,4
0,2
0,0
0123456
789
M e
2+
/1
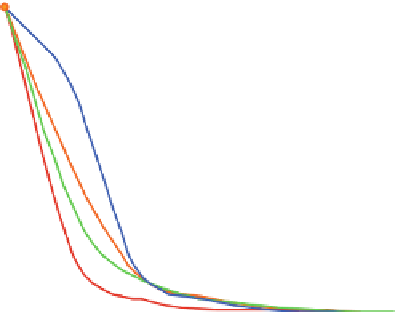
Fig. 12 Fluorescence emission (l
¼
340 nm) of 10 in the presence of different transition metal
exc
10
6
M, TMAOH 1.3
10
5
M and
cations in acetonitrile (
T
¼
20
C). Concentration of 10 6
10
4
M
TBAPF
6
of 1
Ligand 12 provides more interesting results with two possible, yet distinct,
binding stoichiometries and depends on the identity of the metal ion. Cu
2+
,Ni
2+
,
and Pb
2+
give a 1:1 complex, even in the presence of a high cation concentration.
Fluorescence quenching is observed in each case (Fig.
13
) along with a consider-
able shift of the wavelength of the maximum emission for Cu
2+
and Pb
2+
. In this
case, the geometry involves both amino chains in complexation, as indicated above,
with this type of complex influencing the rotation of the biphenyl moiety, which
gives rise to the corresponding fluorescence quenching. In contrast, addition of Cd
2+
and Zn
2+
initially leads to fluorescence quenching with a concomitant red shift, but it
subsequently results in enhanced fluorescence due to the formation of new complexes
with the 1:2 stoichiometries (Fig.
11d
)[
23
].



















































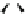






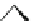























































































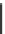

































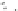





















Search WWH ::

Custom Search