Biomedical Engineering Reference
In-Depth Information
18
16
14
12
10
8
(a)
6
4
2
0
300
400
500
600
λ
(nm)
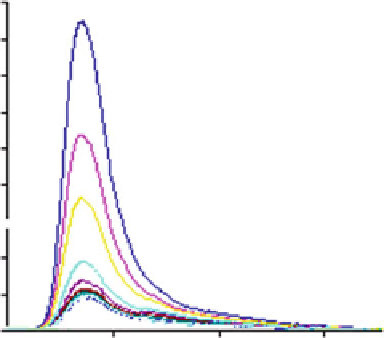
Fig. 9 Fluorescence spectra of 8 after addition of Zn
2+
at 20
C, l
exc
¼
300 nm. The initial ligand
concentration was 10
5
MinCH
3
CN and salt was added until high excess was achieved
Figure
9
shows the quenching experimented by ligand 8 in the presence of
increasing amounts of Zn
2+
. The results obtained with Cd
2+
are very similar.
From the electrochemical point of view, these ligands exhibit clear modifications
in the cyclic voltammetric (CV) data in the presence of transition metal cations.
These modifications are reflected in Fig.
10
and are similar to those observed with
compounds 4 and 5 [
22
].
Thus, oxidation takes place through the nonplanar radical cation; consequently,
peak III totally disappears and peaks II and I overlap. These results demonstrate that
these compounds are able to act as electrochemical sensors for Zn
2+
and Cd
2+
.
However in this case, selectivity is not observed as the behavior with both cations is
very similar.
In order to find selectivity, polyazapodands deriving from TMB were prepared.
These ligands offer the advantage of greater flexibility which, despite diminishing
complex strength, enables a clamp-type complex formation (Scheme
3
).
Thus, compounds 10-12 (Scheme
3
) show marked perturbations in their fluores-
cence spectra, which are associated with complexation by aliphatic amine groups.
Thus, ligand 10 displays a quenching of emission intensity of around 90% after the
addition of 1 equiv. of metal, and little dependence on metal identity. Given the 1:1
stoichiometry of the binding, the most likely geometry of the complex formation is
(a) in Fig.
11
, in which both chains are involved in binding the metal.
Since this complexation hinders the rotation of the two phenyl rings, it accounts
for the fluorescence quenching observed in the presence of metal ions and the
similar behavior noted for all the metals studied (Fig.
12
).
On the other hand, compound 11's behavior strongly depends on pH. For
instance in the case of Zn
2+
, which is a post-transition metal with no ligand field
stabilization energy effects, complexation of the metal is not sufficiently favorable
Search WWH ::

Custom Search