Biomedical Engineering Reference
In-Depth Information
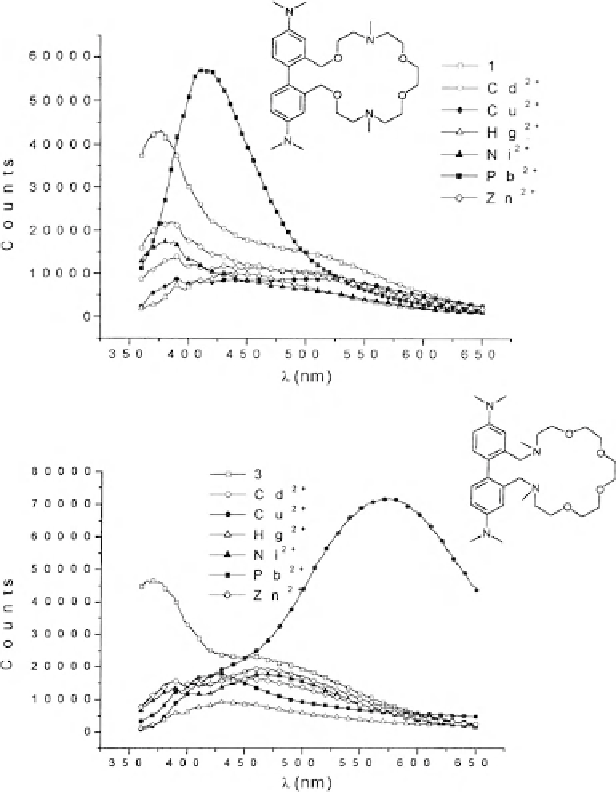
Fig. 4 Emission spectrum of (
left
) 1, and (
right
) 3 in acetonitrile in the presence of Ni
2+
,Cu
2+
,
Zn
2+
,Cd
2+
,Hg
2+
, and Pd
2+
a new band at ca. 570 nm, shifted 200 nm from the emission band of the free
receptor. This new band is obtained only in the presence of Cu
2+
; other metal ions
such as Ni
2+
,Zn
2+
,Cd
2+
,Hg
2+
, and Pb
2+
merely produce a quenching of the 367 nm
emission band. Studies carried out with ligands 1 and 3 have shown a linear
correlation between changes in the optical spectrum and metal ion concentration.
On the other hand, the detection limits were 0.7 ppm for Pb
2+
with ligand 1, and
0.2 ppm for Cu
2+
with ligand 3. Transition metal ions are known to quench
fluorescence very effectively. However, it has also been shown that Cu
2+
,Ni
2+
,
and Pb
2+
can cause fluorescence enhancement in a cryptand-based fluorophore due
to the strong cryptate effect. In addition, similar to the results observed by
Search WWH ::

Custom Search