Biomedical Engineering Reference
In-Depth Information
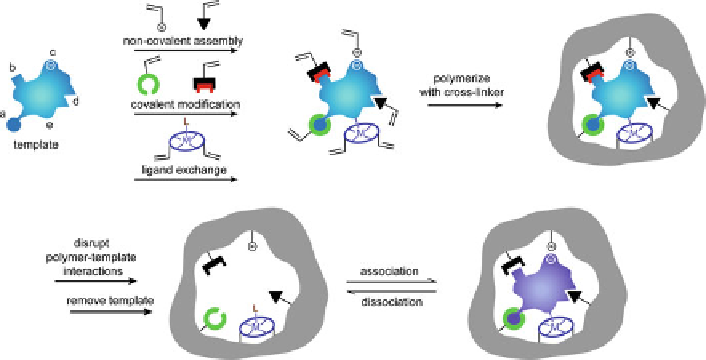
Fig. 1 Schematic representation of molecular imprinting. Template and the polymerizable func-
tional monomer may interact through: (a) reversible covalent bonds, (b) covalently attached
polymerizable binding groups that are activated for non-covalent interaction by template cleavage,
(c) electrostatic interactions, (d) hydrophobic or van der Waals interactions, (e) metal-ion
mediated interactions (adapted with permission from [
30
])
non-covalent interactions. The reaction mixture includes a cross-linker and a
porogenic solvent. Polymerization is initiated either thermally or by UV light,
leading to highly cross-linked polymers. The template is removed from the polymer
by washing, leaving behind binding sites that are both spatially and chemically
complementary to the template molecules, and capable of rebinding either the
template or its structural analogues [
29
,
30
].
In contrast to biomolecules, MIPs are usually stable at low and high pH,
pressures and temperatures (
150
C) [
15
,
31
-
36
]. Moreover, they are less expen-
sive than biomolecules and easier to obtain, and they can be used in organic
solvents. Finally, they can be synthesized for diverse classes of substances, such
as ions [
37
], nucleic acids [
38
], proteins [
39
,
40
], drugs [
41
-
43
], and even yeast
cells and erythrocytes [
44
].
The number of published papers in the MIP area in the last 10 years has tripled,
which reflects the growing interest in these materials [
45
]. However, MIPs are also
burdened with some limitations, mainly connected with the methods of their
production and the final format of the polymer. One such limitation is linked to
MIPs prepared in the “bulk” format, which require grinding and sieving to obtain a
fraction of particles with a narrow range of sizes. This is a lengthy process and is
impractical or unsuitable for many applications [
46
-
48
]. Furthermore free radical
addition polymerization is exothermic and the bulk format prevents efficient heat
exchange with the exterior. This can lead to rapid increases in the temperature of the
polymerization mixture and consequently increased pressure within the reaction
mixture, which may adversely affect MIP properties and can lead to explosions for
<
Search WWH ::

Custom Search