Biomedical Engineering Reference
In-Depth Information
15.6
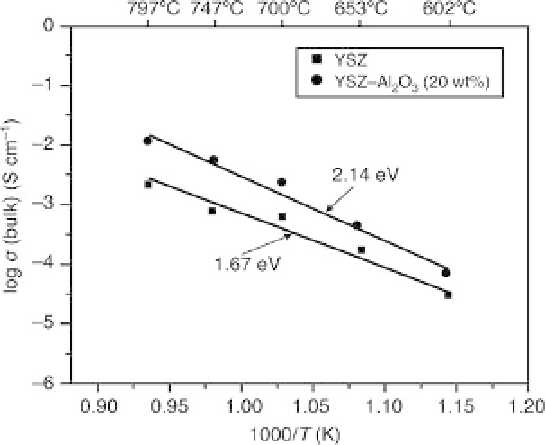
Arrhenius plots of bulk conductivities for yttria-stabilized zirconia
(YSZ) and YSZ-Al
2
O
3
specimens.
15.3.3 Charge carrier and mobility measurements
An ionic conductor responds to an electric field, leading to polarization of
charges. This basic property of ionic conductors is used to calculate the
number of charge carriers and their mobility (Kumar et al., 1994). The
technique basically involves polarizing a specimen using a DC field for a
given period and subsequently monitoring the decay of current resulting
from polarized charges. The polarized nanocomposite acts like a concentra-
tion cell whose potential can be expressed by the Nernst equation:
E
¼
ln C
2
½
=
½
C
1
½
15
:
17
where E is the thermodynamic potential, C
1
is the anodic concentration, and
C
2
is the cathodic concentration.
If the polarized cell is allowed to reach equilibrium, the potential drops to
zero. The area under the current decay curves yields the capacity (
μ
Ah) of
the cell, which is also related to the number of charge carriers (n
i
) displaced
during the application of DC potential. The mobility,
μ
i
, is calculated using
the basic equation of conductivity as expressed by equation 15.5.
Table 15.2 lists the bulk conductivity (
σ
b
), number of charge carriers (n
i
),
and mobility (cm
2
V
1
s
1
) of oxygen vacancies in YSZ and YSZ-Al
2
O
3
specimens at 500, 600, and 700
C. At a given temperature, the YSZ-Al
2
O
3
specimen shows a decreased number of charge carriers as compared with the
YSZ specimen. The ratio n
(YSZ-Al2O3)
/n
YSZ
decreases from 0.95 at 500
8
8
Cto
0.16 at 700
8
C. The decrease in the number of charge carriers by a factor of