Biomedical Engineering Reference
In-Depth Information
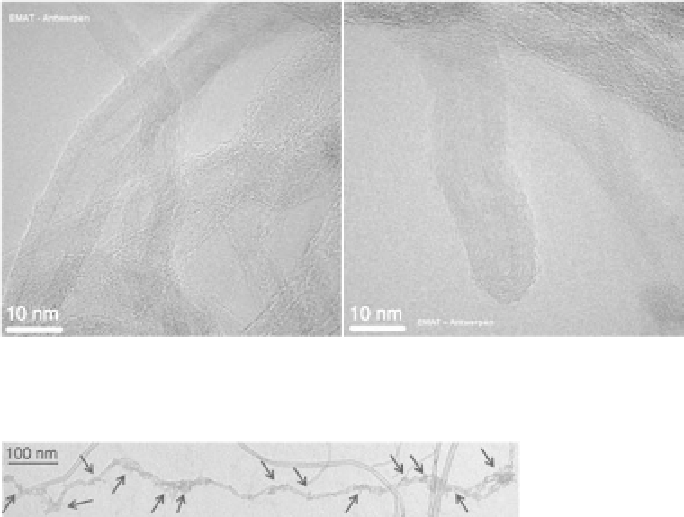
14.14 HRTEM images of CNTs treated in the post-discharge chamber
of Ar + N
2
μ-wave plasma (Ruelle et al., 2008).
14.15
TEM image of one CNT with PCL islets resembling a pearl-
necklace structure (Ruelle, 2009).
surfaces and atomic nitrogen species, without the side-effects of irradiation
or high energy particle interaction at the CNT surface.
With the addition of H
2
in the post-discharge, an efficient and selective
grafting of primary amine groups was developed. Actually, two-thirds of
grafted nitrogenated functional groups were primary amine groups, as
determined by XPS after (trifluoromethyl)benzaldehyde derivative reaction
(Ruelle, 2009). These grafted amine groups have been used as initiation sites
for promoting the ring opening polymerization (ROP) of
-
CL) yielding polyester-grafted CNT nanohybrids (Ruelle et al., 2007). The
morphology of the recovered nanohybrids has been characterized by TEM,
showing that PCL islets, and so the initiator primary amines were
homogeneously dispersed along the CNT sidewalls (Fig. 14.15).
Moreover, the nanohybrids displayed the best dispersion ability in chloro-
form, a good PCL solvent, in comparison to pristine and amino-CNTs.
The CNT-g-PCL nanohybrids, mixed with free PCL chains, presented a
high degree of CNT pre-disaggregation and were thus used as nanofillers in
PCL; pristine and amino-CNT-filled nanocomposites were also prepared as
comparative materials. TEM images showed that slightly disrupted CNT
bundles are present in the CNT-filled PCL nanocomposites while CNTs are
homogeneously dispersed when CNT-g-PCL nanohybrids are used (Fig.
14.16). Electrical conductivity measurements of the PCL nanocomposites
showed a similar percolation threshold (
ε
-caprolactone (
ε
~
0.35 wt%)
for each PCL