Biomedical Engineering Reference
In-Depth Information
14.2.2 Sidewall functionalization
The other approach for covalent grafting reactions is the 'sidewall
functionalization', which consists of grafting chemical groups through
covalent reactions onto the
-conjugated skeleton of CNTs (Hirsch, 2002).
Unlike defect-site chemistry, which takes advantage of defects already
present in the CNT structure, the direct covalent sidewall functionalization
is associated with a change in hybridization from sp
2
to sp
3
. This
simultaneous loss of conjugation of CNTs influences their physical
properties, and more particularly their electrical conductivity, depending
on the density of functionalization.
First, covalent sidewall functionalization was carried out on the basis of
well-developed grafting chemistry on fullerenes whose reactivity depends
strongly on the curvature of the carbon framework. However, the sidewall
reaction chemistry of CNTs differs from that of fullerenes as the chemical
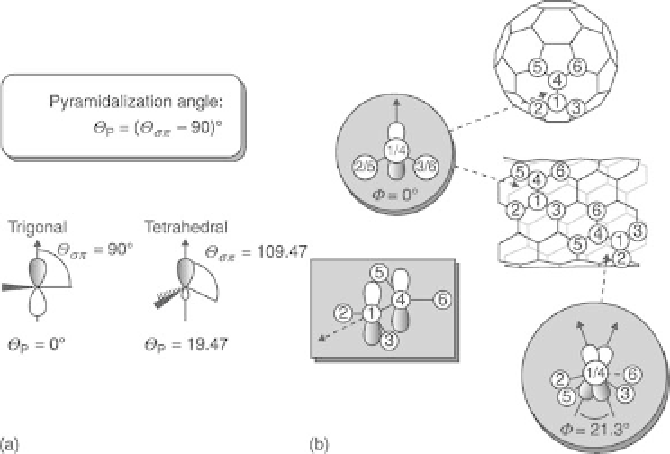
reactivity in carbon systems arises from two factors that induce local strain:
the pyramidalization at the carbon atoms and the
π
-orbital misalignment
between adjacent pairs of conjugated carbon atoms (Chen et al., 2003). The
fullerene structures and the CNT end-caps present a pronounced
pyramidalization of the carbon atoms further improving chemical reactions.
In the CNT sidewalls, the pyramidalization strain is not as acute and, thus,
π
π
-orbital misalignment has a greater influence on sidewall chemical
reactivity (Hamon et al., 2001). In Fig. 14.3, the reactivity of the C-C
bond in CNT structure is presented in function of its angle to the tube
14.3
(a, b) Pyramidalization angles (
Θ
P
) and the
π
-orbital misalignment
angles (
) along C1-C4 in the CNT framework and its capping fullerene
C
60
(Hirsch and Vostrowsky, 2005).
Φ