Biomedical Engineering Reference
In-Depth Information
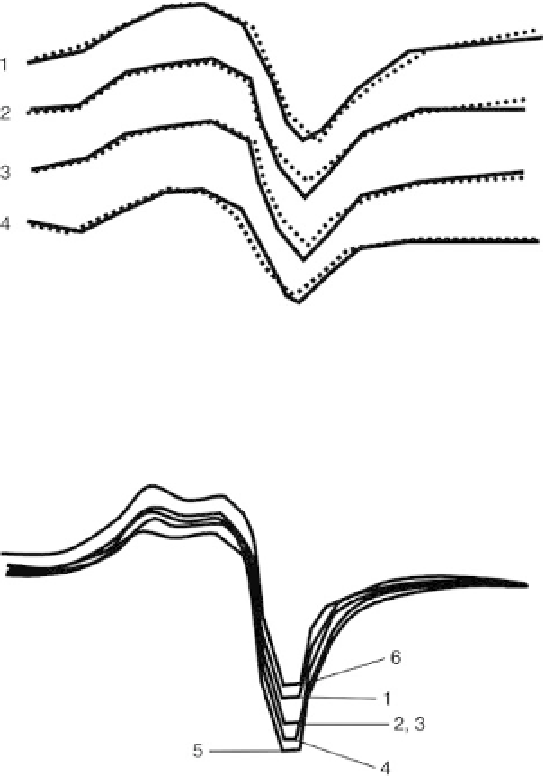
Cu
2+
(I) EPR spectra of SC Y
1
Ba
2
Cu
3
O
7
x
ceramics (
9.16
i
= 92.0 K);
reference, dotted curves; nanocomposites, solid line curves. Curve 1,
15% PS and 85% ceramic. Curve 2, 15% PMMA and 85% ceramic. Curve
3, 20% PE and 80% ceramic. Curve 4, 15% copolymer of ST and MMA
and 85% ceramic [24].
T
Cu
2+
(I) of SC Y
1
Ba
2
Cu
3
O
6.97
ceramic (curve 1) and
nanocomposites with SHMPE: curve 2, 1% SHMPE; curve 3, 3% SHMPE;
curve 4, 5% SHMPE; curve 5, 10% SHMPE; curve 6, 20% SHMPE [24].
9.17
It is known that acrylamide (AAm) complexes of metal nitrates of the first
transition group are able to polymerize at frontal regimes. The essence of
frontal polymerization is in localized heating of the sample edge, initiating
polymerization [28]. The heat evolved is transmitted to neighboring layers
by a heat-conductance mechanism, where, in turn, polymerization begins.
Thus, the heat wave front propagates over the entire volume. As metal-
containing monomers are able to polymerize frontally, complexes like
(AAm)
4
(H
2
O)
2
(MO
3
)
2
(M=Mn, Co, Y, Cu, etc.) could be used. A previous
investigation showed that frontal polymerization of AAm complexes in the
presence of SC ceramic is possible only within a limited temperature range.
It was shown experimentally that the lower temperature limit for carrying
out the reaction (100
C) is given by the stability of frontal polymerization
upon the propagation of vertical heat waves from up to down. This sharply
8