Biomedical Engineering Reference
In-Depth Information
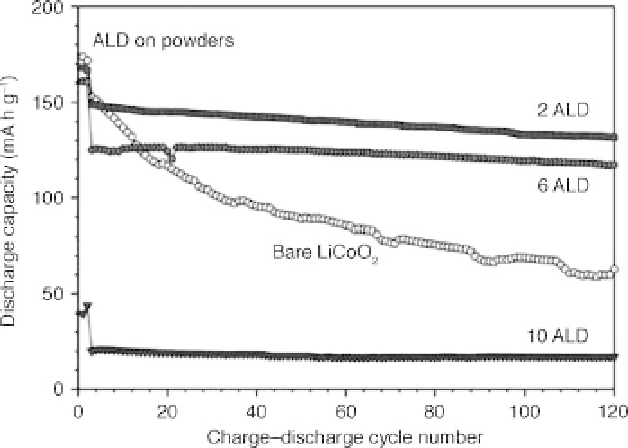
8.8 Charge-discharge cycle performance of electrodes fabricated
using bare LiCoO
2
powders and the Al
2
O
3
ALD coated LiCoO
2
powders
using 2, 6, and 10 ALD cycles (Jung et al., 2010a). Reproduced by
permission of the Electrochemical Society.
increasing number of ALD cycles. The reduction in electron conductivity
could result in slower charge-discharge kinetics. The Al
2
O
3
ALD film could
also reduce Li-ion conductivity. It is important to note that the charging to
4.5 V is atypically high for a LiCoO
2
battery system. This is another
important feature of the coating, that the safety aspect of the battery system
is greatly improved during non-standard operation such as overcharging.
One set of experiments by Jung et al. (2010b) supported the conclusion of
the hindrance of electron transport in the Al
2
O
3
film resulting from the
complete ALD coverage on the active material powder. Five cycles of ultra-
thin alumina ALD films were deposited directly on natural graphite (NG)
composite electrodes. For comparison, NG electrodes prepared with Al
2
O
3
ALD coatings on powder and bare NG were tested using charge-discharge
cycling at an elevated temperature of 50
C. The bare NG displayed a
relatively rapid decay in reversible capacity versus the number of charge-
discharge cycles. In contrast, the capacity retention was dramatically
improved by performing only five cycles of Al
2
O
3
ALD directly on the
electrode. The charge-discharge capacity retention for the electrode coated
with five Al
2
O
3
ALD cycles was 98% for 200 charge-discharge cycles,
normalized to the reversible capacity at the third charge-discharge cycle,
with negligible kinetic hindrance. Clearly, direct deposition of ALD films on
finished electrodes is another viable technique to produce ALD-enabled
battery materials, which is another route to protecting the surface of the
8