Biomedical Engineering Reference
In-Depth Information
dip-coating
[57,58]
, electrochemical deposition
[59]
, or electrostatic atomization spray techniques
[60]
.
In-vitro
study with rabbit mesenchymal stem cells on nanostructured HA coatings indicated
enhanced osteoblast response and biocompatibility of such surfaces
[61]
. Recent
in-vivo
study in rab-
bits showed increased bone formation on nanoHA-coated titanium implants and significant influence
on early bone formation compared to uncoated implants after 4 weeks of healing
[62]
. These coatings
can be further functionalized with osteoinductive biomolecules.
In-vitro
study with SaOS-2 human
osteoblast-like cells on nanoHA coatings functionalized with collagen by sol-gel technique showed
increased osteoblast activity
[63]
. Silanization using APTES chemistry has also been utilized in cova-
lent attachment of RGD peptide on HA-coated titanium substrates.
In-vitro
study with osteoprogeni-
tor cells isolated from human bone marrow stroma cells showed enhanced osteoblast adhesion and
function on such surfaces
[64]
.
Different forms of calcium phosphate (octa-, di-, tri-, and tetra-calcium phosphate) have been
coated on titanium surface
[65,66]
. Plasma-spraying technique has been utilized to coat calcium phos-
phate on titanium cylinders.
In-vivo
implantation in dog femur and histological evaluation revealed
that these coatings enhanced bone formation with excellent bone-to-implant surface contact
[67]
.
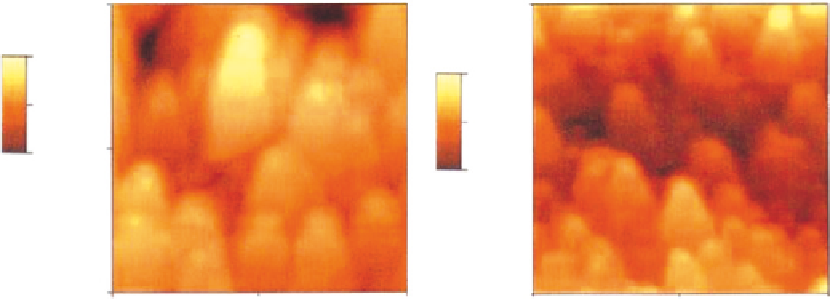
Pulsed laser deposition (PLD) technique has also been employed to fabricate calcium phosphate coat-
ings on titanium substrates (
Figure 6.4
). These types of coatings exhibited much higher adhesive
strength with substrates than conventional plasma-sprayed coatings
[68]
.
In-vitro
study with human
osteoblasts showed that the coatings enhanced osteoblast adhesion, proliferation, and differentiation.
Titanium has also been coated with biphasic calcium phosphate particles using grid-blasting tech-
nique to increase the roughness of titanium implants and enhance the attachment of mouse osteoblasts
and increase the alkaline phosphatase activity
[69]
. A recent approach involved placing of Ti-6Al-4V
discs in concentrated SBF forming a dense layer of amorphous calcium phosphate
[70]
. This layer
served as seeding surface and the discs were placed in supersaturated calcium phosphate solution
that produced a coating up to 100 μm thick. BMP-2, a potent osteoinductive agent, was passively
absorbed on to the coating. The titanium discs with calcium phosphate-BMP-2 coating were implanted
2000nm
2000nm
174nm
76nm
1000nm
1000nm
0.00nm
0.00nm
0nm
0nm
0nm
1000nm
2000nm
0nm
1000nm
2000nm
(A)
(B)
FIGURE 6.4
Atomic force microscopy (AFM) images of the coating surface showing tips of B100nm high (A) crystalline
and (B) amorphous calcium phosphate coatings produced by PLD
[68]
.