Biomedical Engineering Reference
In-Depth Information
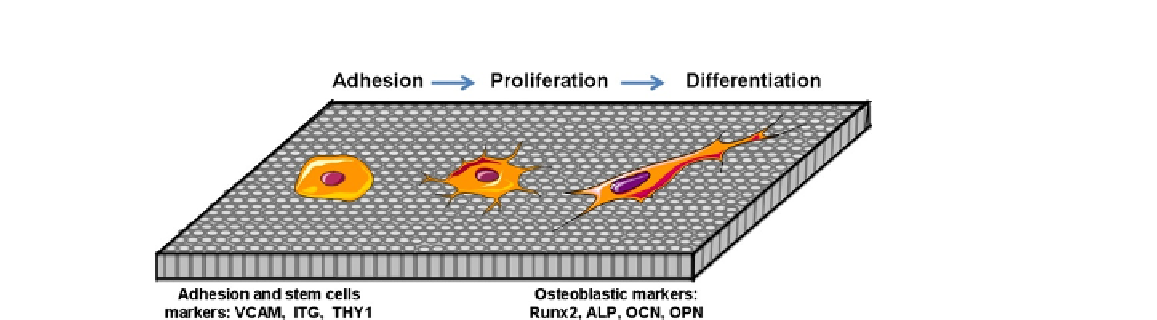
FIGURE 5.5
Scheme showing the adhesion, proliferation, and differentiation of MSCs on
nanostructured surfaces. The adhesion of stem cells is characterized by the expression
of cell surface markers (VCAM, ITG, THY1) while phenotypic markers (Runx2, ALP,
OCN, OPN) are specific to their osteoblastic differentiation (OCN: osteocalcin; OPN:
osteopontin).
from other cell types by two important characteristics. First, they are unspecialized cells able to
renew themselves through cell division, sometimes after long periods of inactivity. Second, under cer-
tain physiologic or experimental conditions, they can be induced to become tissue- or organ-specific
cells with special functions. MSCs have high proliferative and multi-potent capacity leading to dif-
ferentiated cells under the guidance of various cues or niches. MSCs are conventionally defined as
adherent, non-hematopoietic cells expressing markers such as CD13, CD29, CD44, CD54, CD73,
CD90, CD105, and CD166, and being negative for CD14, CD34, and CD45
[28,29]
. While originally
identified in the bone marrow
[30]
, MSCs have been extracted from numerous tissues including adi-
pose
[31,32]
, heart
[33]
, dental pulp
[34]
, peripheral blood
[35]
, cord blood
[36]
. One of the major
properties of MSCs is their ability to differentiate into various cells like adipocytes
[37]
, chondro-
cytes
[31]
, osteoblasts
[38]
, neurons
[39,40]
, muscles
[40,41]
, hepatocytes
[42]
in vitro
after treat-
ment with induction agents.
5.4.2
Migration, Adhesion, and Proliferation
The integration of implant with neighboring bone and gingival tissue depends on successful crosstalk
between surrounding tissue and implant surface. The challenge in dental implant research is the capa-
bility of the surface to guide cells colonization and differentiation. Cell migration, adhesion, and pro-
liferation on implant surfaces are a prerequisite to initiate the tissue regeneration (
Figure 5.5
). Authors
have shown that some factors present in tissues and secreted during the inflammatory phase are able
to attract MSCs to the injured site
[43,44]
. MSCs migration and proliferation were stimulated
in vitro
by many growth factors including PDGF
[45,46]
, EGF
[46,47]
, VEGF
[48]
, TGF-β
[45,49]
, BMP-2
and BMP-4
[45,48]
. These factors are certainly released in the injured sites by cells involved in tis-
sue healing. Furthermore, plasma clot serve as storage to fibrin molecules and release system for a
variety of bioactive factors including growth factors that attract and differentiate MSCs into specific
lineages
[50-52]
. The platelet factors are well-known to stimulate the proliferation of MSCs
[53]
. The
formation of a clot matrix with a potent chemo-attractive factor like PDGF, EGF, or fibrin may further