Biomedical Engineering Reference
In-Depth Information
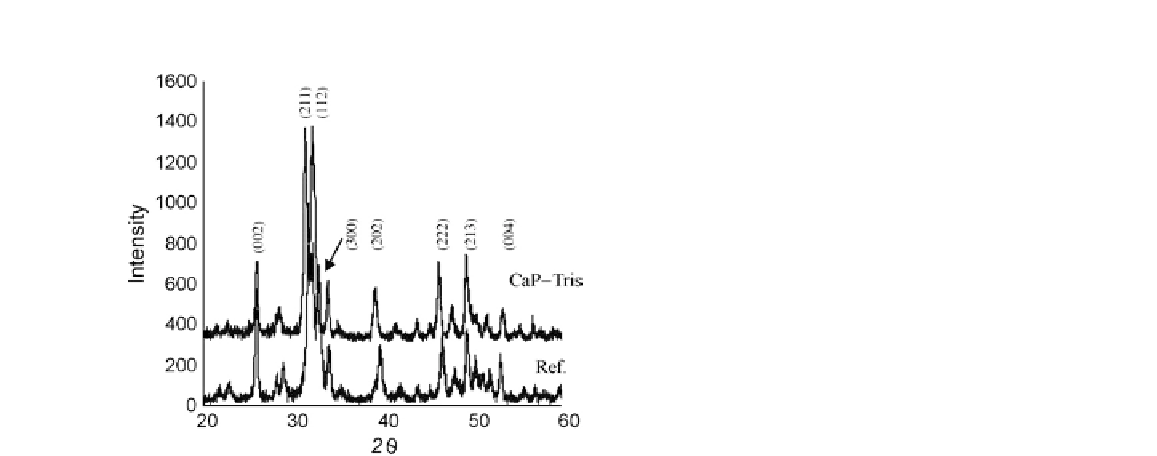
FIGURE 18.8
XRD patterns of HA reference and synthesized using
CaP-Tris solution
[48]
.
being the most widely used value. Liquid mercury has a high surface tension; usually its value is
taken to be 485 mN/m (485 dyne/cm)
[50]
. High-pressure mercury porosimeters can normally attain
maximum pressures of 207 MPa (30,000 psia) or 414 MPa (60,000 psia). The measurable pore size
ranges from a maximum of 360 μm to a minimum of 6 nm (for the 30,000 psia system) or 3 nm (for
the 60,000 psia system)
[50]
. In principle it would be possible to explore in the pore range below
3 nm if a porosimeter with sufficiently high pressures could be made
[49]
. In practice BET surface
area analysis is normally employed for area analysis where pore size is below 2 nm. In mercury poro-
simetry, an apparatus is used to evacuate the sample and then to surround the sample with mercury.
Evacuation is achieved by exposing the sample to a vacuum. The sample to be analyzed is contained
inside a penetrometer, which is a long glass capillary tube, the sample end being bulb shaped. Using
the vacuum control on the filling apparatus, gases and vapors are removed from the sample. The vac-
uum valve is closed and the penetrometer titled so that the stem end is immersed in mercury. The vent
control valve is then slowly opened such that air fills the mercury chamber. Mercury is forced up the
capillary stem and into the bulb. The filled penetrometer is then removed and inserted in the high-
pressure porosimeter for pore analysis.
The construction of the glass penetrometer is key to pore measurement. A metal sheath fits over
the capillary section. A metal seal attaches to the sample end of the penetrometer as a base elec-
trode. The construction is thus mercury-glass-metal, or conductor-insulator-conductor. In this way
a coaxial capacitor is created
[50]
. The capacitance changes as a result of the change in mercury
level within the penetrometer. The mercury level will change as porous samples are filled with mer-
cury under increasingly high pressure. In a similar way, a normal mercury thermometer will change
mercury level, indicating a temperature change. Some porosimetry systems operate on the basis of a
wire dipped remotely into the mercury; the change in electrical resistance of the wire being used as a
means of measuring the volume of mercury taken up by the pores
[49]
.