Biomedical Engineering Reference
In-Depth Information
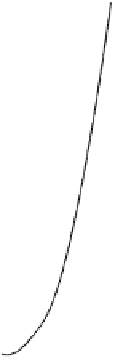
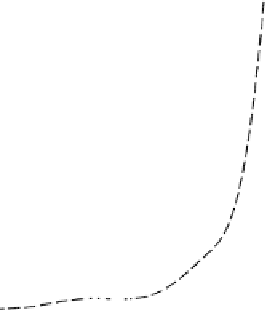
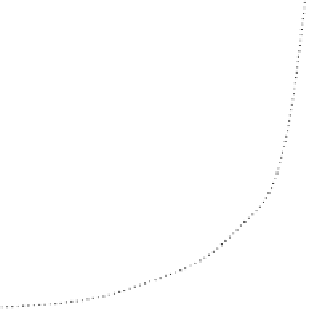
FIGURE 8.9
Absorbance-time curves for the browning of glucose+glycine in buffered
solutions (a) in the absence of inhibitor and in the presence of (b) mercaptoethanol, (c) S(IV),
and (d) glutathione and the dipeptides. The curves for reactions in the presence of gly-cys,
leu-cys, val-cys, aba-cys, and glu-cys are within the limits of the horizontal bars. Reaction
conditions: 0.5 mol l
-1
acetate buffer, pH 5.5, 55°C, [thiol] or [S(IV)] = 20 mmol l
-1
,
[glucose] = 1 mol l
-1
, [glycine] = 0.5 mol l
-1
. Reproduced from Edwards, A. S., Wedzicha,
B. L., and Wedzicha, B. L.,
Food Chem.
, 51, 389, 1994. © 1994 Elsevier Science Ltd. With
permission.
financial support from the Biotechnology and Biological Sciences Research Council
(formerly the Agricultural Research Council and then the Agricultural and Food
Research Council) through research fellowships and equipment grants. The most
recent contributions have arisen through generous financial support from the Com-
mission of the European Communities, Agriculture and Fisheries (FAIR) specific
RTD program, CT96-1080, “Optimization of the Maillard Reaction: A Way to
Improve the Quality and Safety of Thermally Processed Foods”. The research does
not necessarily reflect its views and in no way anticipates the Commission's future
policy in this area.
REFERENCES
1.
Wedzicha, B. L.,
Chemistry of Sulphur Dioxide in Foods
, Elsevier Applied Science
Publishers, London, 1984.
2.
Taylor, S. L., Higley, N. A., and Bush, R. K., Sulfites in foods: uses, analytical
methods, residues, fate, exposure assessment, metabolism, toxicity and hypersensi-
tivity,
Adv. Food Res.,
30, 1, 1986.
3.
Rose, A. H. and Pilkington, B.J., Sulfite, in
Mechanisms of Action of Food Preservation
Procedures,
Gould, G. W., Ed., Elsevier Science Publishers Ltd, Barking, 1989, 201.





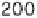
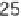


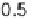



















































Search WWH ::

Custom Search