Biomedical Engineering Reference
In-Depth Information
nonlinearities in Arrhenius plots of reaction rates may result from (1) first-order
phase transitions (e.g., melting of solid fat, which may increase mobility of potential
reactants in the resultant liquid phase); (2) crystallization of amorphous sugars may
release water and affect the proportion of reactants in the solute-water phase;
(3) freeze-concentration of solutes in frozen foods may increase concentration of
reactants in the unfrozen solute matrix; (4) reactions with different activation ener-
gies may predominate at different temperatures; (5) an increase in a
w
with increasing
temperature may enhance reactions; (6) partition of reactants between oil and water
phases may vary with temperature depending on phase transitions and solubility;
(7) solubility of gases, especially of oxygen, in water decreases with increasing
temperature; (8) reaction rates may depend on pH, which also depends on tempera-
ture; (9) loss of water at high temperatures may alter reaction rates; and (10) protein
denaturation at high temperatures may affect their susceptibility to chemical reactions.
It has been well established that water as a plasticizer has a significant effect
on molecular mobility and probably on rates of quality changes above a critical,
temperature-dependent a
w
or water content. A chemical reaction requires sufficient
mobility of reactants and products in addition to the driving force, e.g., temperature
or concentration, of the reaction or change in quality. Slade and Levine
6
suggested
that diffusion in amorphous foods is related to viscosity and, therefore, governed by
the glass transition. According to this assumption, the rate of a reaction is controlled
by viscosity and diffusion and it may be assumed that below T
g
the rate of a reaction
can be extremely slow. At temperatures above T
g
, diffusivity increases as viscosity
decreases and in some cases the temperature dependence of the reaction rate may
follow the WLF-type temperature dependence. It is likely that in low-moisture and
frozen foods, a change in the rate constant of a diffusion-controlled reaction or
constants of deteriorative reactions at temperatures typical of food storage are rela-
tively low and only minor changes of activation energies can be observed as food
materials are transformed from the solid, glassy state into the supercooled liquid state.
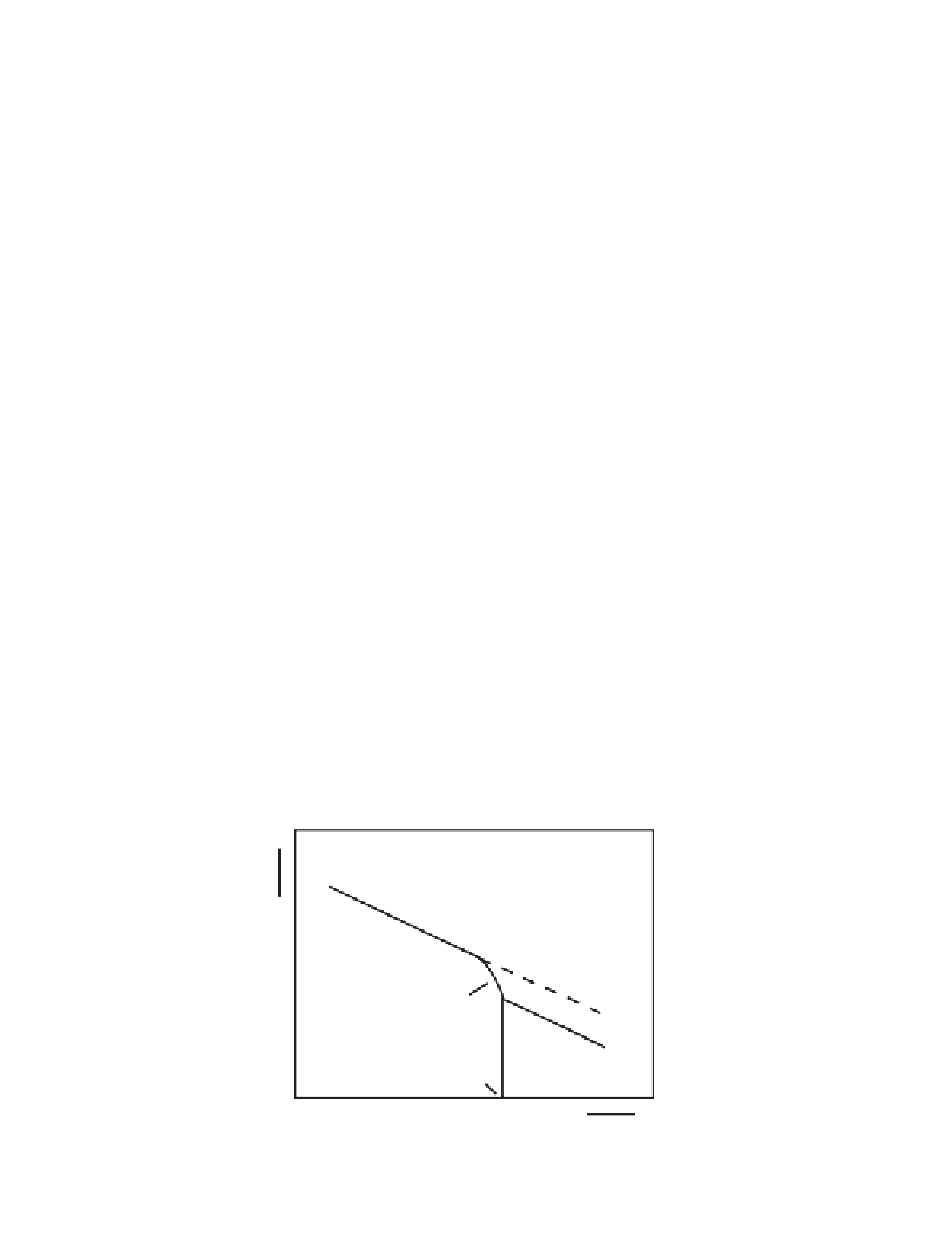
Glass Transition
1/T
g
-1
(TEMPERATURE)
FIGURE 1.11
The effect of glass transition on the temperature dependence of reaction rate
constants, k, of diffusion-controlled reactions, as may be observed from the Arrhenius plots.



Search WWH ::

Custom Search