Biomedical Engineering Reference
In-Depth Information
but the mechanism of the formation of melanoidins appears very different, with those
derived from ascorbic acid and glycine having a much larger number of 3-deoxypen-
tosulose-derived subunits per glycine residue, than those from the pentoses.
73
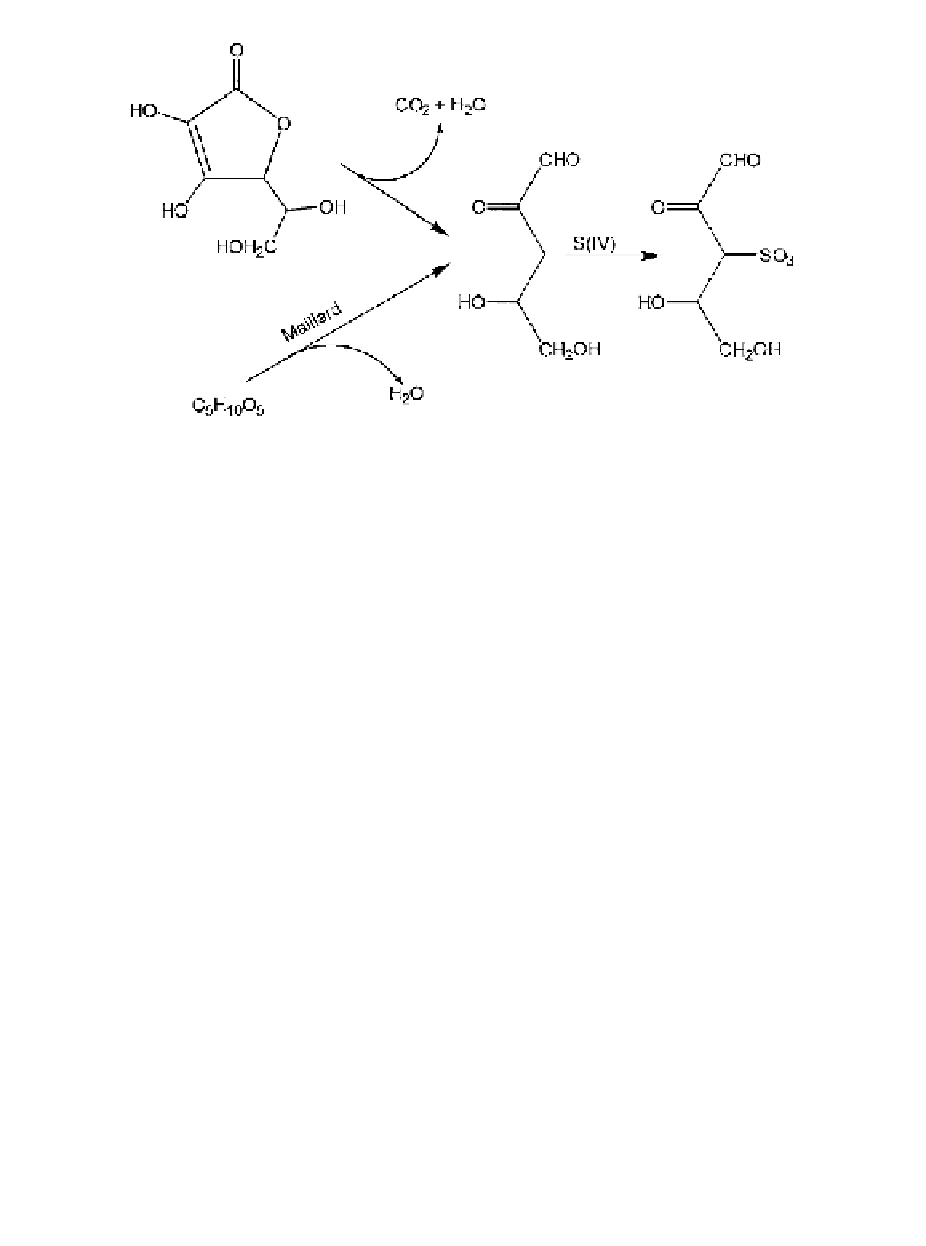
In the presence of air, ascorbic acid is converted to dehydroascorbic acid; this
reaction takes place by way of an unusually stable free radical intermediate, mon-
odehydroascorbic acid, which is very difficult to trap or react with conventional
antioxidants. In food and model systems, it is usually impossible to avoid the
oxidation of ascorbic acid unless oxygen is strictly excluded from the system. While
dehydroascorbic acid exhibits vitamin C activity, it is much more susceptible to
browning and its presence is regarded as undesirable. The use of ascorbic acid as
an inhibitor of enzymic browning results in the coupled oxidation of the vitamin by
the quinone intermediates; the widely seen practice of adding ascorbic acid to apple
and pear juice for fermentation is probably of no consequence with respect to the
browning of ascorbic acid in long-term storage because these beverages are also
treated with S(IV) which protects also against the browning of dehydroascorbic acid.
The mechanism of the inhibition of dehydroascorbic acid browning is still not well
understood, but dehydroascorbic acid forms a stable monohydroxysulfonate (
K
=
5.7
10
-4
mol l
-1
), which should be much less reactive.
74
On the other hand, dehy-
droascorbic acid decarboxylates and dehydrates to L-xylosone which also forms a
stable carbonyl-S(IV) adduct (
K
= 1.4
×
The kinetics of the irreversible binding of S(IV) in the ascorbic acid-S(IV)
reaction, under anaerobic conditions, suggest that S(IV) catalyze the conversion of
ascorbic acid to 3-deoxypentosulose (DP) or some other intermediate which reacts
with S(IV) to give DSP.
75
Indeed, at millimolar concentrations of S(IV), and at
pH 3 to 5, the rate of the catalyzed reaction far exceeds the rate of spontaneous
decomposition of the vitamin. The possibility that S(IV) is able to increase the rate
of hydrolysis of the lactone moiety of ascorbic acid, which is regarded as the first
step in the mechanism of its decomposition to DP, cannot be tested directly on
ascorbic acid, but it is found that the rate of hydrolysis of
×
-gluconolactone (which
can be measured using optical rotation) is, indeed, increased by S(IV).
75
δ
Search WWH ::

Custom Search