Biomedical Engineering Reference
In-Depth Information
[
]
=
[
]
()
glucose
glucose
SIV
[
HS
,
(8.16)
+
[
]
K
i.e., for a glucose concentration of 1 mol l
-1
approximately half of the S(IV) is
reversibly bound.
52,53
As the reaction proceeds, the fraction of the total S(IV) which
is bound increases with time, indicating that carbonyl compounds that bind S(IV)
more strongly than glucose are being formed. It has been shown that, in the glu-
cose-glycine-S(IV) reaction, the principal S(IV)-binding component is DSH
(hydroxysulfonate dissociation constant = 4 mmol l
-1
).
53-55
The kinetics of the irre-
versible binding of S(IV), the formation of hydroxysulfonates, and the relationship
of this mechanism to the route taken by the Maillard browning reaction are described
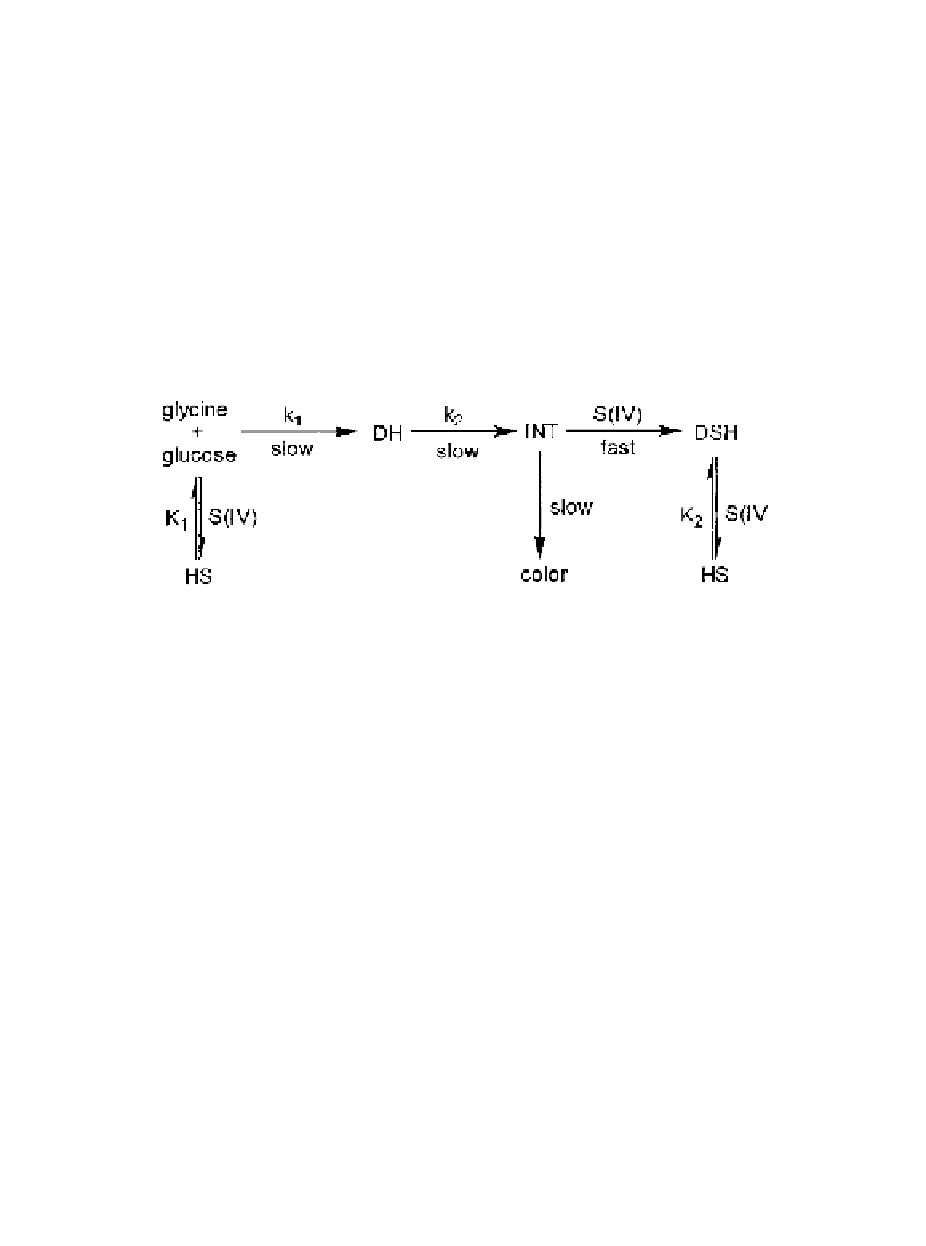
by the following model:
where INT is an unspecified intermediate common both to browning and the reaction
with S(IV),
k
1
and
k
2
are rate constants, and
K
1
and
K
2
are equilibrium constants.
This kinetic model has been tested rigorously and found to apply to a wide range
of sugars and amino acids, and under a variety of reaction conditions.
56-62
The
unspecified intermediate could well be DDH and the success of S(IV) as an inhibitor
of browning rests with its ability to compete effectively for this intermediate. In
model systems, e.g., containing aqueous solutions of glucose (1 mol l
-1
), amino acid
(0.5 mol l
-1
), and S(IV) (0.05 mol l
-1
), the concentration of S(IV) becomes limiting
in the conversion of the intermediate to DSH, only after some 75% of the additive
is irreversibly lost. In this situation a very high proportion of what remains is
reversibly bound and the concentration of free S(IV) to react with the intermediate
is very small. This explains why the reaction becomes dependent on S(IV) concen-
tration. On the other hand, most evidence concerning the loss of S(IV) from foods
suggests that the kinetics are of first order with respect to this additive.
The high molecular weight colored products in Maillard browning (melanoidins)
are partially bleached by the addition of S(IV). Those (with M
r
> 12,000) formed in
the glucose-glycine reaction combine irreversibly with S(IV) such that one C
C
bond for every two glucose-derived residues is lost; it is envisaged that here SO
3
2-
could be adding to an
-unsaturated carbonyl compound or a Schiff base derived
from it.
63
As the melanoidin reacts with S(IV), the sulfur to carbon ratio of the product
increases, but this is related linearly to its absorbance. Melanoidins are polymers and
this result suggests that the chromophores in these products behave independently
64
and they are not the extensively conjugated structures suggested by some authors.
α
,
β
Search WWH ::

Custom Search