Biomedical Engineering Reference
In-Depth Information
nearly vertical suggesting the absence of a reversible interaction between SO
2
and
other species involved in the transport process.
26
Further evidence for the non-
involvement of a conventional protein-assisted membrane transport process comes
from the fact that none of the reagents, carbonyl cyanide
m
-chlorophenylhydrazone,
DNP, iodoacetamide, or
p
-chloromercuribenzoate inhibit the transport of SO
2
,
despite being able to inhibit lysine transport under the same conditions. Cell death
occurs after a loss of ATP
29
either as a result of the activation by S(IV) of an ATP-
hydrolyzing enzyme, the inactivation of glyceraldehyde-3-phosphate dehydroge-
nase, or by using up ATP to pump H
+
out of the cell in order to restore the pH within
the cell. The last of these possibilities also applies to the majority of the carboxylic
acid food preservatives. However, S(IV) inhibit a wide range of metabolic enzymes
by reaction with disulfide bonds (
Figure 8.6
,
reaction III), coenzymes (as illustrated
in Figure 8.6 reaction IV for NAD
+
) and cofactors, substrates and intermediates in
enzyme reactions.
3
Similarly, the cleavage of disulfide bonds may result in the
denaturation of structural proteins.
Perhaps the best known interaction between components of living systems and
S(IV), and of considerable significance in foods, is the addition of HSO
3
-
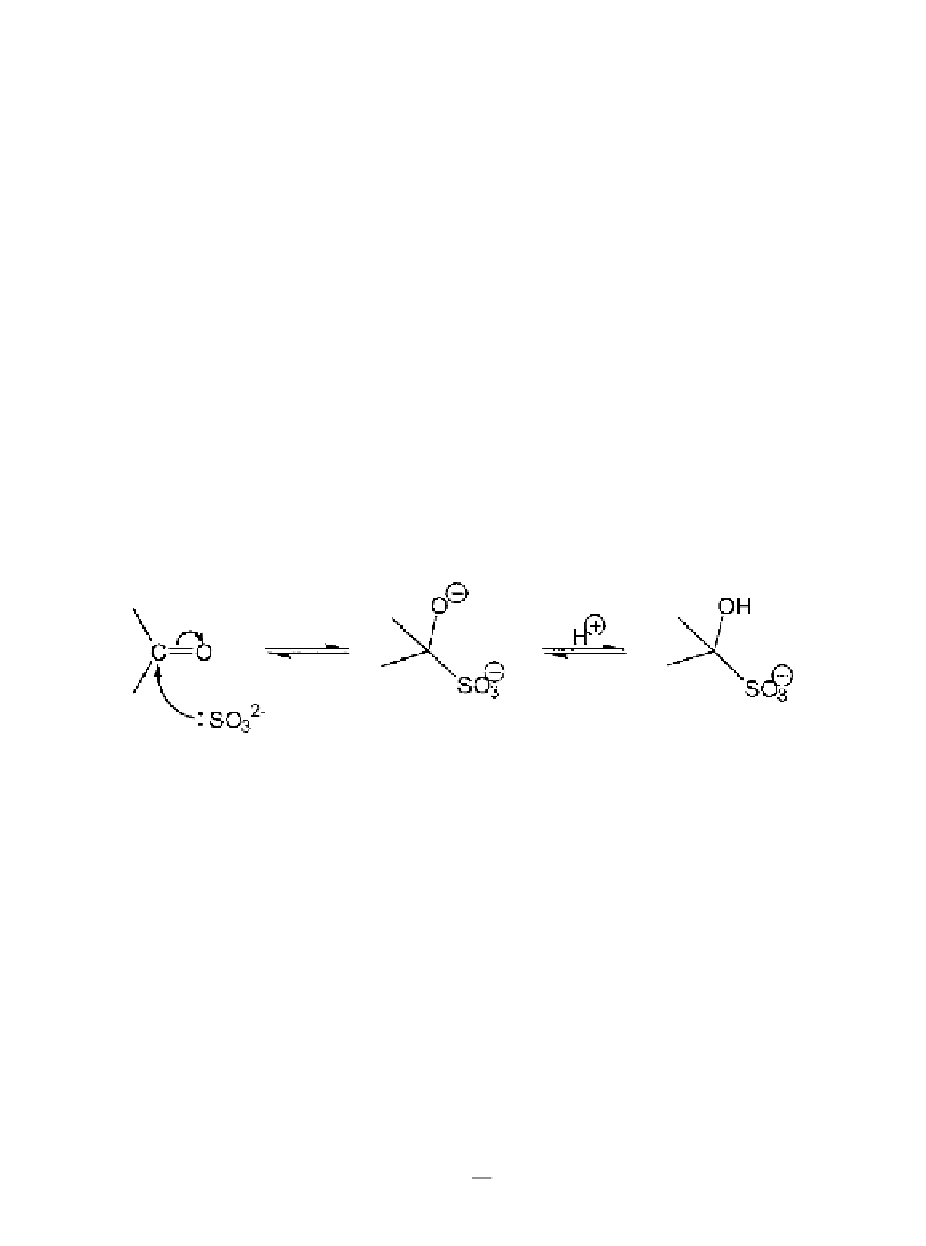
to carbonyl
groups (Figure 8.6, reaction I). Its mechanism
30,31
involves two steps; first there is
nucleophilic attack at the carbonyl group by SO
3
2-
after which the reaction is
completed by the addition of H
+
, as follows:
The rate of the forward reaction depends on pH in so far as this determines the
concentration of the nucleophile, which is approximately proportional to [H
+
] when
the pH is well below the p
K
a
value of HSO
3
-
, i.e., within the pH range of most
foods. On the other hand, the first step in the decomposition of hydroxysulfonates
is the ionization of the OH group (p
K
a
= 10.7); the rate of the reverse reaction is
inversely proportional to [H
+
] over this pH range. The equilibrium constant for the
dissociation of hydroxysulfonate (HS) is given by
[
]
[
]
()
SIV C
HS
=
0
K
=
(8.11)
[]
and corresponds to the ratio of rate constants for the reverse and forward reactions,
k
r
and
k
f
, respectively, i.e.,
k
k
K
=
r
(8.12)
f
Search WWH ::

Custom Search