Biomedical Engineering Reference
In-Depth Information
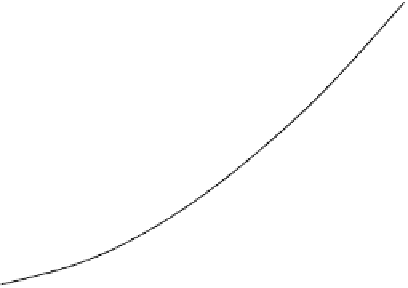
FIGURE 8.5
The effect of concentration of non-electrolyte on p
K
2
of SO
2
·H
2
O at 30°C
and [S(IV)] = 50 mmol l
-1
.
, Ethanol; ∆, Glycerol;
▫
, PEG400; ∇, Sucrose. Reproduced from
Wedzicha, B. L. and Goddard, S. J.,
FoodChem
., 40, 129, 1991. © 1991 Elsevier Science
Ltd. With permission.
TABLE 8.1
Calculation of the Percentage (mol/mol) of HSO
3
-
Remaining in Equilibrium with S
2
O
5
2-
, in a Solution
Initially Consisting of HSO
3
-
at a Concentration
s
s
/mol l
-1
0.5
1.0
1.5
2.0
2.5
3.0
3.3
I
/mol l
-1
0.5
2
1.0
7
1.6
6
2.2
8
2.9
3
3.6
0
4.0
1
mol% HSO
-
93.7
86.3
78.9
72.0
65.7
60.1
57.0
I
is the ionic strength at equilibrium.
Reproduced from Wedzicha, B. L. and Goddard, S. J.,
Food Chem
., 40, 121,
1991. 1991 Elsevier Science Ltd. With permission.
and the expected variation in solute activity coefficients with ionic strength.
16
How-
ever, increased concentration also leads to anion-cation (ion-pair) interactions
becoming significant. Thus, even the singly charged alkali-metal ions interact sig-
nificantly with doubly charged anions such as SO
3
2-
and S
2
O
5
2-
, as illustrated in
aqueous solvents and humectants are added to aqueous solutions of S(IV). For
example, the formation constant of the ion-pair NaS
2
O
3
-
is 4.8, 71.4, and 143 mol
-1
l in water,
17,18
44 and 50 wt% ethanol,
19
respectively; these results are expected to
be applicable to SO
3
2-
because the thiosulfate ion shows solute-water interactions


































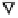
















































Search WWH ::

Custom Search