Biomedical Engineering Reference
In-Depth Information
K
1
H
+
K
1
K
2
H
+
K( )
app
=
K
H
1
+
-----------
+
-------------
(8.9)
2
[]
[]
where
K
1
and
K
2
are the first and second dissociation constants of sulfurous acid.
The value of Henry's constant is also sensitive to the presence of solutes which can
form complexes with SO
2
. The SO
2
molecule is able to accept electrons from
nucleophiles; complexes, such as SO
2
I
-
, SO
2
Br
-
,
10
and SO
2
Cl
-
,
11
have been identified
and their stabilities determined. In principle, amines (e.g., amino acids, lysine res-
idues on proteins) and other nitrogenous bases are expected to form such complexes.
However, at pH values sufficiently low for significant concentrations of SO
2
to be
present, these bases are protonated and do not behave as nucleophiles; hence, the
significance of such interactions in the food matrix is likely to be small. Nevertheless,
the stability of SO
2
Cl
-
(dissociation constant = 7.1 mol l
-1
) is sufficient for there to
be a reduction in the vapor pressure of SO
2
above solutions of the gas,
11
upon the
addition of NaCl, as illustrated in
Figure 8.1
.
In the normal pH range of food, pH 3 to 6, the principal species is HSO
3
-
,in
equilibrium with small but pH-sensitive amounts of SO
2
·H
2
O and SO
3
2-
.These minor
species are responsible for the preservative action and chemical reactivity of the
additive. However, it is important to appreciate that, in some instances, the p
K
values
of SO
2
·H
2
O are sensitive to the composition of the medium, other than its pH. The
8.3, in accordance with theoretical predictions based on the variation of solute
FIGURE 8.1
The effect of [Cl
-
] on the value of Henry's constant
K
H
for SO
2
in the
headspace above a solution of the gas in water at 25°C. The error bars represent standard
deviations obtained from 15 replicate experiments. Reproduced from Wedzicha, B. L. and
Webb, P. P.,
Food Chem
., 55, 338, 1996. © 1996 Elsevier Science Ltd. With permission.








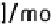






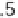





































































Search WWH ::

Custom Search