Biomedical Engineering Reference
In-Depth Information
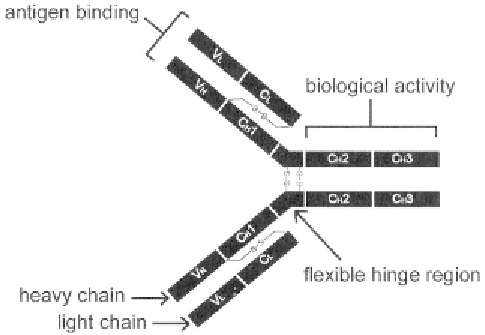
Figure 7.
Schematic of an IgG antibody
class to which the antibody belongs. Figure 7 shows a two dimensional
model of the typical 4 chain structure of an IgG antibody. The heavy
(H) and light (L) chains are made up of variable (V) and constant (C)
region domains that have similar 3-dimensional structures. Note that the
V regions of the H and L chains, which make up the antigen binding site,
are on a different end of the molecule from the C regions, which are
responsible for the biologic activity of the antibody. The identification of
the 4 chain structure of antibodies was a tour-de-force of basic protein
chemistry, and earned for Gerald Edelman and Rodney Porter the Nobel
Prize in Medicine in 1972.
Antibodies are relatively stable proteins and can be modified for use
in research or as diagnostic tools. Antibodies can be labeled with ra-
dioactive tracers such as or covalently conjugated with biotin or
fluorescent dyes, and still retain antigen specificity and biologic func-
tions. Consequently, antibodies can be used to reveal the subcellular
location of a protein, identify a protein band on a western blot, or iden-
tify a protein that binds a particular promoter sequence. They can be
used to affinity purify a protein from a complex mixture of proteins, or be
used to quantitate the levels of a hormone. The uses are limited only by
the imagination of the investigator!
Nevertheless, polyclonal antibodies have one drawback: they must be
continually produced by immunizing experimental animals and purified
from their serum. This limits the amount of any antibody that can be iso-
lated, and limits their distribution to other investigators, or their commer-
cialization (although many polyclonal antibodies are produced for sale
to the scientific community). This changed in 1975, when George Köhler
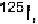
Search WWH ::

Custom Search