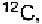Biomedical Engineering Reference
In-Depth Information
Chapter 1
DETECTION AND ANALYSIS OF PROTEINS
A.
Introduction
Proteins are the body's building blocks. They are the second most
abundant part of our bodies, comprising about 20% of our weight (the
most abundant constituent, water, accounts for 70%). Proteins make
up muscles, most of our enzymes are proteins, and the antibodies
that protect us from pathogens are glycoproteins, or proteins that also
contain carbohydrate sidechains. Proteins are biopolymers that consist
of various mixtures of the 20 amino acids. The word “protein” is de-
rived from a Greek root meaning “of first importance”. Proteins were
discovered in 1838 by Jöns Jacob Berzelius (1779-1848), a Swedish
chemist. Berzelius also developed a system of chemical notation that
is essentially the same basic system used today. Berzelius is one of
three chemists who are considered the fathers of modern chemistry.
The other two chemists are Antoine Levoisier (1743-1794) and John
Dalton (1766-1844). Levoisier was the first to articulate and experi-
mentally demonstrate the idea of the conservation of matter. He used
quantitative methods to measure products of chemical reactions, allow-
ing the composition of compounds to be determined with considerable
accuracy. Levoisier also had the distinction of losing his head (literally!)
during the French Revolution.
John Dalton made numerous contributions to the field of chemistry.
He formulated the atomic theory, which proposed that elements were
composed of atoms in fixed amounts and, therefore, each element had a
fixed mass. His name is inextricably linked to measurements of weights
of proteins. The basic unit is a “Dalton”, or Da, which is defined as
one-twelfth the mass of the most abundant isotope of carbon,
which is equal to grams. The association of Dalton's
name with precise measurements of atomic mass seems ironic to me,
in that Dalton was said to be a brilliant mind but not a particularly good