Biomedical Engineering Reference
In-Depth Information
Congenic strains of mice were instrumental in helping to understand
the nature of genes that encode histocompatibility antigens and to de-
fine the Major Histocompatibility Complex (MHC). The MHC is called
H-2 in mouse, and HLA in humans, and encodes a number of closely
linked genes that are responsible for the rejection of tissues (kidneys,
heart, skin, etc.) that are transplanted between two unrelated indi-
viduals. MHC differences trigger profound immunological responses,
and are the reason that immunosuppressive drugs are required when
transplanting tissues between any two persons except identical twins.
These genes also help guide the development of receptors for foreign
antigen on one of the major classes of lymphocytes, T lymphocytes.
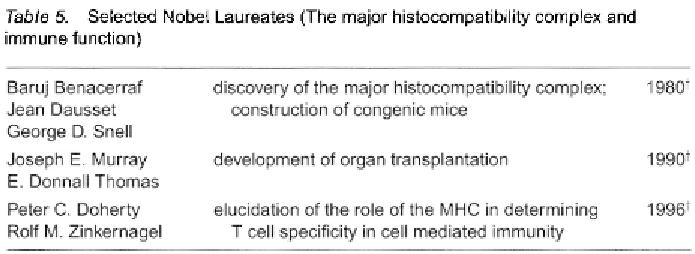
George Snell developed the idea of generating congenic mice for iden-
tifying the genes involved in tissue rejection, and for this work was
awarded the Nobel Prize in 1980 (which he shared with Baruj Benacerraf
and Jean Dausset) for the discovery and characterization of the MHC
(Table 5).
Congenic strains of mice have also been used to determine the impor-
tance of a gene (and closely linked genes) to the onset of disease. They
have also contributed significantly to studies that have mapped genes
that contribute to complex diseases, such as systemic lupus erythe-
matosis, an autoimmune disease that plagues hundreds of thousands
of people, especially women. Generating and testing these mice takes
patience: it can take three years or more to produce one individual mouse
line.
Speed congenic strains of mice
Speed congenics utilize genomic scanning methods to help reduce
the number of generations of backcrossing necessary to generate a
congenic line. In this method, donor mice having the gene of interest
Search WWH ::

Custom Search