Biomedical Engineering Reference
In-Depth Information
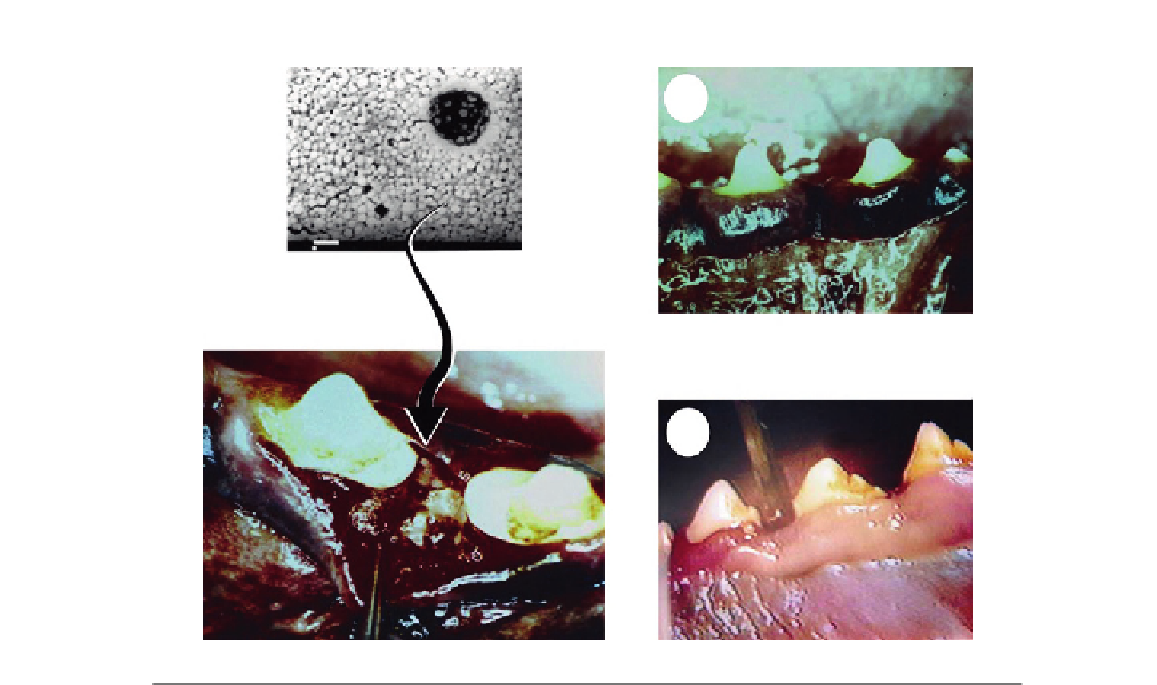
(A)
(B)
FIGURE 23.8
Experimental sites after the administration of TCS-loaded PLGA nanoparticles in dogs: (A) 8 and (B) 15 days.
This enhanced bactericidal activity of HLE/PLGA nanoparticles may be due to the bioadhesive
property of the PLGA biopolymer, which remains on the bacterial cells for a prolonged period,
thus extending the drug action.
A drug-delivery system for dental applications was proposed by Bak´ et al.
[27]
. Biocompatible
nanoparticles were obtained by free radical initiated copolymerization of the monomers
2-hydroxyethyl methacrylate and polyethylene glycol dimethacrylate in aqueous solution. This
polymerization yielded a well-dispersible white powder material composed of nanoparticles with a
size between 50 and 180 nm suitable for incorporation into a hydrogel matrix and to design new
drug-delivery media for dental applications.
It is well recognized that minocycline is one of the broad-spectrum antibiotics frequently used
for the treatment of periodontitis and related infections in periodontal diseases
[53,54,64]
. Recently,
different methods, such as single emulsion (oil/water, modified oil/water and oil/oil) and double
emulsion
solvent evaporation (water/oil/water), ion pairing, and nanoprecipitation were used to
prepare both PLGA nanoparticles and PLGA with polyethylene glycol (PEG) nanoparticles
(PEGylated PLGA nanoparticles) containing minocycline
[28]
. Almost all of the nanoparticles pre-
pared from PLGA and PEG
PLGA under different conditions were less than 500 nm with a spheri-
cal shape and a smooth surface. The nanoparticles obtained by solid/oil/water ion pairing showed