Biomedical Engineering Reference
In-Depth Information
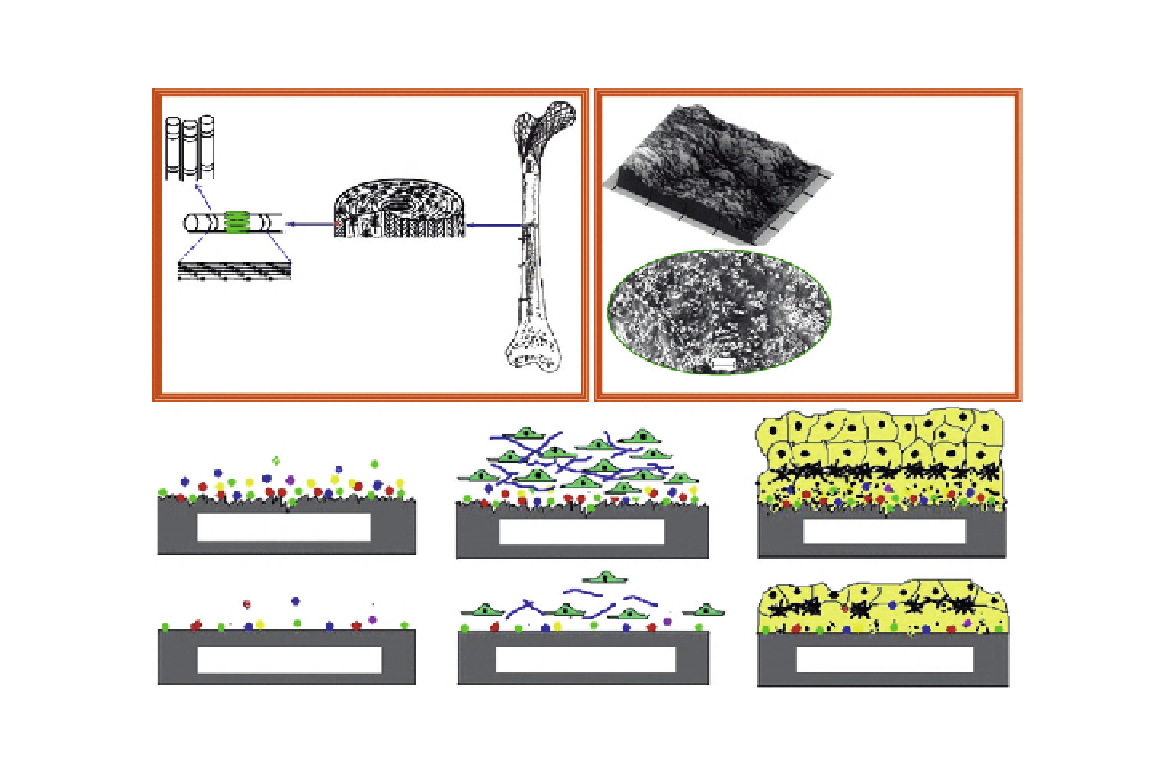
(A)
Nanostructured bone
(B)
Nanomaterials
Biomimetic
nanomaterials have
improved cytocompatible,
mechanical, or electrical
properties.
Collagen fibers
Large fibers
Osteons
Layers in the osteon
Unique nanotopography
and surface chemistry
may increase protein
adsorption, osteoblast
functions and rapidly
induce osseointegration
Microfibril with
hydroxyapatite
Compact bone
Nanocrystalline hydroxyapatite hydrogel scaffolds
(C)
Nanophase material
Nanophase material
Nanophase material
Conventional material
Conventional material
Conventional material
Protein adsorptions on
substrates immediately
Osteoblast attachment and
proliferation (0-3 days)
Osteoblast differentiation and
bone remodeling (>21 days)
FIGURE 20.1
The biomimetic advantages of nanomaterials. (A) The nanostructured hierarchal self-assembly of bone.
(B) Nanophase titanium (top, the atomic force microscopy image) and nanocrystalline HA/HRN hydrogel
scaffold (bottom, the SEM image). (C) Schematic illustration of the mechanism by which nanomaterials may
be superior to conventional materials for bone regeneration. The bioactive surfaces of nanomaterials mimic
those of natural bones to promote greater amounts of protein adsorption and efficiently stimulate more new
bone formation than conventional materials.
Adapted from Ref.
[5]
. Reprinted with permission from Elsevier.
ratio and unusual chemical synergistic effects. Nanosized HA is expected to have a better bio-
activity than coarser crystals
[20
22]
. Similar tendencies have been reported for other nano-
ceramics including alumina, zinc oxide, and titania. Osteoblast adhesion increased by 146% and
200% on nanophase zinc oxide (23 nm) and titania (32 nm) compared to microphase zinc oxide
(4.9
m), respectively
[23,24]
.
Commercial formulations (nano-bone) have also been developed and extensively used in clinic.
nanOss
μ
m) and titania (4.1
μ
bone void filler from Angstrom Medica Inc. is considered as the first nanotechnological
medical device receiving clearance by the US Food and Drug Administration (FDA) in 2005.
s