Biomedical Engineering Reference
In-Depth Information
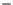
1400
pg/mL CS
pg/mL nCS
pg/mL nCS
+
alg
1200
1000
800
600
400
200
0
0 h
2 h
24 h
7 days
−
200
Time
FIGURE 19.5
Release of rhBMP-2 from nCS alone, nCS
10% alginate in comparison to CS. Disks were fabricated from
the scaffold materials with rhBMP-2 (Prospec, Rehovot, Israel), added at a final concentration of 1
1
g/disk.
BMP-2 released from the disks at 37
C was measured with a specific immunoassay (Quantikine Kit, R&D
Systems). Values are the mean
μ
standard deviation of four samples in each group.
6
delivery agent in various in vivo situations where bone formation is the desired end function.
Although our in vitro data suggest that nCS releases only a small percentage of absorbed BMP-2,
an effective dose cannot be predicted since the in vivo conditions in a particular site may signifi-
cantly alter the kinetics of release and availability of the growth factor at that site. The complexities
involved in the delivery of growth factors to in vivo sites in tissue engineering strategies have been
reviewed
[54,55]
and it must be recognized that extrapolation from in vitro studies to a particular
in vivo model is extremely limited.
Therefore, as an extension of these in vitro studies, most recently, we also tested nCS in the rat
calvarial defect model, which is a well-studied model that allows in vivo testing of the bone regen-
erative ability of a material
[56,57]
. All protocols used here were approved by the Institutional
Animal Care and Use Committee of State University of New York at Buffalo, New York, USA.
Basically the technique involved creating an 8 mm critical size defect using a low-speed hand piece
and trephine drill in the calvaria of adult male Sprague-Dawley rats. The cranial defect created in
each rat was then filled with a constant amount (100 mg) of either nCS alone, or nCS mixed with
10 or 50% alginate with and without PDGF-BB or rhBMP-2. In some other rats, the defect was
filled with Capset
s
which was a commercially available conventional-sized CS, or GEM21
s
,a
commercially available (Osteohealth) mixture of beta TCP and PDGF, or DFDBA (demineralized
freeze-dried bone allograft, commercially available, Dentsply). In the negative control group, the
defects were left untreated. There were a minimum of four animals in each treatment and control
group. In all animals, the overlying tissues were closed in layers with resorbable 4
0 Vicryl
s
sutures. As shown in
Figure 19.6
, radiographic analysis using Image J software to measure bone
filling in the calvaria from the margin of defect after 2 weeks of treatment, revealed that were no