Biomedical Engineering Reference
In-Depth Information
Recently, two acute toxicity tests (oral and dermal) using the Re-doped IF-MoS
2
NP were con-
ducted
[50,51]
. The objective of the first study was to assess the acute oral toxicity of Re-doped
IF-MoS
2
NP (Re:IF-MoS
2
) following single administration of 2000 mg/kg, by oral gavage to mice,
using prespecified fix doses, based on the identification of the dose(s) mortality and/or moribund
status of the animals. The results showed no mortality in any of the animals throughout the entire
14-day study period. No noticeable clinical signs in reaction to dosing were evident in any of the
animals. Mean group body weight gain at the end of the 14-day study period was noted in all
groups. No gross pathological findings were evident in any of the animals at the time of their
scheduled necropsy. Histopathologic findings revealed no treatment related changes in all the
organs examined. Based on the lack of observed adverse reactions Re:IF-MoS
2
may be regarded as
not causing acute toxicity risk through this route of administration and is classified as hazard
Category 5 according to the Globally Harmonized Classification System
[50]
(
Figures 13.13 and
13.14
).
A second acute dermal toxicity of Re-doped MoS
2
was assessed on the basis of the testing proce-
dure recommended by the OECD Guideline for the Testing of Chemicals. A single limit dose level
corresponding to 2000 mg/kg of Re:IF-MoS
2
was topically applied for 24-h exposure duration to
mice, for the purpose of evaluating health hazards likely to arise from short-term exposure in consid-
eration of its projected use as a coating on medical devices. The results showed no mortality prior to
the scheduled sacrifice. No clinical signs were observed in any of the animals at the immediate time
postdosing. The body weight gain of all animals at the end of the 14-day observation period was
within the normal limits. No abnormalities were detected in any of the animals at the time of their
scheduled necropsy, at 14-days postdosing. Based on the results obtained following a single dermal
application of Re:IF-MoS
2
dose level corresponding to 2000 mg/kg, it may be concluded that
Re-doped MoS
2
NP do not represent an acute toxic risk by this route of administration
[51]
.
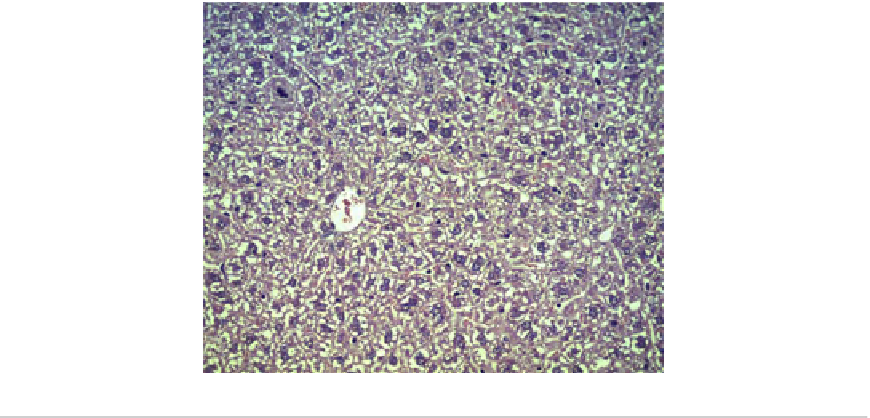
FIGURE 13.13
Liver centrilobular region. No abnormality detected 14 days after single administration of 2000 mg/kg of
Re-doped IF-MoS
2
nanoparticles (Hematoxylin and eosin (H&E),
20).
3