Biomedical Engineering Reference
In-Depth Information
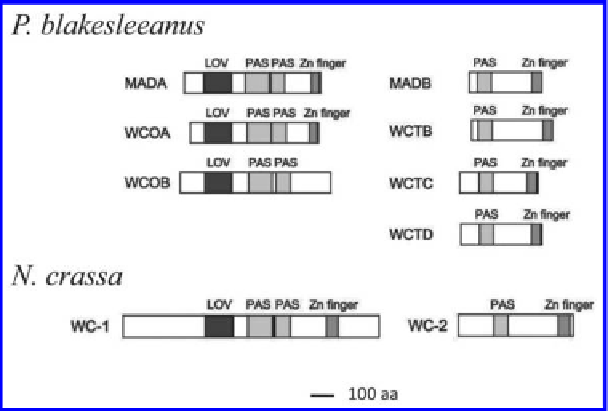
Figure 4.
Comparison of WC proteins from Zygomycete and Ascomycete fungi. The
fi gure shows WC-1 and WC-2 proteins from representatives of the Ascomycota (
N. crassa
)
and the Zygomycota (
P. blakesleeanus
). Flavin chromophore-binding domain (LOV),
protein-interaction domains (PAS) and Zn fi nger domains are indicated.
where the WC1 protein MCWC-1C may act as a photoreceptor protein for
photocarotenogenesis and MCWC-1A may act as a photoreceptor protein
for phototropism (Navarro et al. 2001, Silva et al. 2006). All the
Zygomycete
WC-1 proteins contain a fl avin-binding domain (LOV) supporting their role
as blue-light photoreceptors (Idnurm et al. 2006, Kubo 2009, Sanz et al. 2009,
Silva et al. 2006). In all the analyzed
Zygomycete
fungi multiple
wc
genes
have been reported, suggesting that the presence of the
wc
gene repertoire
was generated before the divergence of the different Zygomycete groups
from a common ancestor. Zygomycete
wc
genes probably arose after gene
duplication events, as shown by the similarities of their genomic structure
(Corrochano and Garre 2010). In
Mucor
a functional analysis of
wc-1
genes was performed (Navarro et al. 2001, Nicolás et al. 2003), indicating
that the main photobiology roles depend on WC-1A and WC-1C as the
photoreceptors for phototropism and photocarotenogenesis, respectively
(Silva et al. 2006). The existence of WC complexes in Zygomycetes was
analyzed in
Phycomyces
where two-hybrid assays and co-expression
analyses in
E. coli
showed an interaction between MadA and MadB. The
absence of any additional interaction between
Phycomyces
WC proteins in
yeast two-hybrid assays suggests that the Mad complex must be the main
photoreceptor complex in
Phycomyces
(Sanz et al. 2009).
A search of mutants specifi cally affected in photocarotenogenesis led
to the identifi cation of
picA
and
picB
(Lopez-Diaz and Cerdá-Olmedo 1981)
and an unrelated search found a defective photocarotenogenesis in a
pim