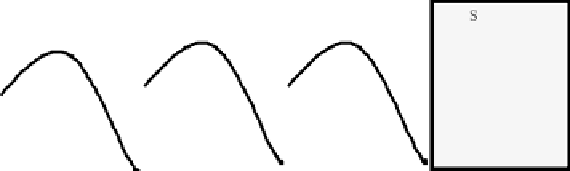Biomedical Engineering Reference
In-Depth Information
enzyme(s) used, the degree of hydrolysis (DH), the amino acid sequence
of the protein substrate and the reaction conditions.
Protein functionality
In nutrition applications a high DH is wanted but a high concentration of
free amino acids (caused by exoproteases) not (e.g. limiting osmolarity in
sports drinks, infant or clinical nutrition). To that end di- or tripeptides
are most desirable. Therefore, a proteolysis process consisting of an
endoprotease followed by a di- or tri-peptidyl-aminopeptidase (DPAP,
TPAP) is a preferred combination. In special cases limited hydrolysis is
needed. As depicted in Fig. 4 limited hydrolysis of protein will improve
physical chemical properties such as foaming or emulsifi cation. Common
endoproteases will only cause limited proteolysis (DH 5-10%) when they
are stopped by inhibition or heat denaturation. Both methods are often
not feasible nor allowed in food processing. An elegant way of achieving
limited proteolysis is using a very specifi c endoprotease capable of
cleaving exclusively next to one amino acid. Such enzymes need not be
inactivated but they stop automatically, due to lack of cleavable peptide
bonds. The proline specific endoprotease identified in the
A. niger
genome is an example of such a specifi c endoprotease (Edens et al. 2005).
Proteolysis of proteins is applied for the following reasons:
(1)
changing of physical chemical characteristics as:
Figure 4.
General relations of various physical chemical protein characteristics and degree
of hydrolysis.