Biomedical Engineering Reference
In-Depth Information
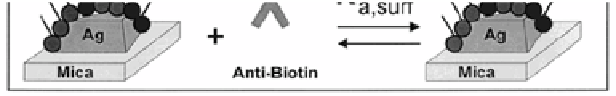
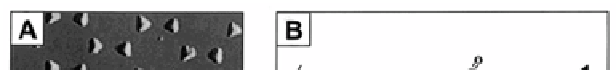
Figure 4.6
LSPR nanobiosensor. (A) Tapping mode AFM image of the
Ag nanoparticles (nanosphere diameter,
D
= 390 nm; mass
thickness,
d
m
= 50.0 nm Ag on a mica substrate). Scan area,
3.0 μm
2
. Scan rate between 1 and 2 Hz. After solvent annealing,
the resulting nanoparticles have in-plane widths of ~100 nm
and out-of-plane heights of ~51 nm. (B) Surface chemistry
of the Ag nanobiosensor. A mixed monolayer of (1) 11-MUA
and (2) 1-OT is formed on the exposed surfaces of the Ag
nanoparticles followed by the covalent linking of (3) biotin
(B) to the carboxyl groups of (1) 11-MUA. (C) Schematic
representation of anti-biotin (AB) binding to a biotinylated Ag
nanobiosensor fabricated by NSL on a mica substrate. Reprint
with permission from ref. 44, Copyright 2003, American
Chemical Society.
The biosensing capability of the nanostructures fabricated by
NSL has been compared with the conventional PSPR sensor.
45
Although the PSPR sensors exhibit signiicantly higher RIS, the
shorter electromagnetic ield decay length associated with NMNPs
provides the LSPR sensors with enhanced sensitivity in surface
binding.
48
As a result, their overall sensitivities in biosensing are
approximately equivalent.
49
Alternatively, Tamiya and coworkers focused their attention on
the fabrication of gold-capped nanoparticle layer substrates (see
























Search WWH ::

Custom Search