Biomedical Engineering Reference
In-Depth Information
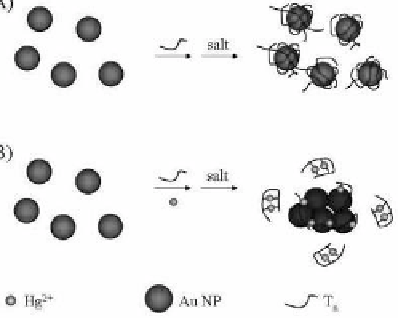
Selective sensing of Hg
2+
using DNA-conjugated Au NPs is
practical via thymine-Hg-thymine (T-Hg
2+
-T) coordinatination.
101
Poly-T
n
ssDNA (
n
= 33, 50, or 80) and Au NPs (13 nm diameter) in
the presence of salt are used for the detection of Hg
2+
ions based
on Hg
2+
-DNA complexes inducing the aggregation of Au NPs (see
Fig. 3.12). Random coil DNA molecules adsorb onto Au NP surfaces
through electrostatic attraction. In the presence of salt, poly-T
n
ssDNA
on the surface of Au NPs remains in a random coil structure as a
result of the electrostatic repulsion between DNA molecules. Owing
to the high negative charge density of DNA on each Au NP surface,
monodisperse Au NPs exist in the salt solution. Upon formation
of Hg
2+
-DNA complexes through T-Hg
2+
-T coordination, the
conformation of the poly-T
n
ssDNA changes to folded structures. As a
result of the decreased zeta potential on each Au NP and the reduced
degree of electrostatic repulsion between Au NPs, aggregation of the
Au NPs occurs, and hence, the color of the solution changes from red
to purple in a process that is detectable by the naked eye. The LOD
for Hg
2+
at a signal-to-noise ratio of 3 is about 250 nM.
(A)
(B)
Figure 3.12
Schematic representation of Hg
2+
nanosensors. Reprinted
with permission from ref. 101.
A label-free, sensitive, selective, and simple enzyme colorimetric
assay using unmodiied Ag NPs is practical for the determination of
the activity of enzymes,
102
based on the fact that the dispersed Ag NP
solution is yellow whereas the aggregated Ag NPs solution is pale red
(or brown). Enzymatic reactions concerning ATP dephosphorylation

















Search WWH ::

Custom Search