Biomedical Engineering Reference
In-Depth Information
conjugation, are attractive. Bifunctional chemicals containing SH or
NH
2
groups at one of their ends are favorable because they can form
strong Au-S and Au-N bonds on the surfaces of Au NPs. For example,
thiol-modiied oligonucleotides are used to functionalize Au NPs
for the speciic detection of nucleic acid sequences in biological
samples. When the functionalization of Au NPs with biomolecules
such as proteins, peptides, and amino acids is necessary, bifunctional
chemical such as thiols, imides, and biotin are more favorable. For
example, Au NPs functionalized with sulfo-
N-
hydroxysuccinimide
can react with primary amines, allowing any protein bearing a
primary amine to be linked to Au NPs. Biotin can also be conjugated
to Au NPs to detect streptavidin through speciic interactions of
biotin-streptavidin.
58,59
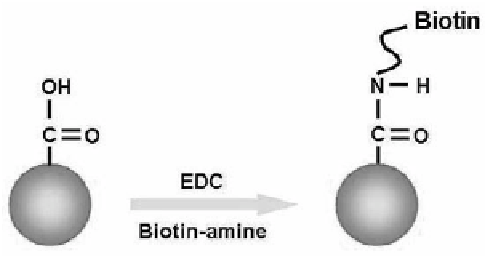
Figure 3.2 displays one representative
example for the functionalization of Au NPs with biotin.
60
The surface
of Au NPs is irst modiied with a layer of poly(acrylic acid). A biotin
molecule in biotin-amine, which has an amine group at one end,
anchors to the carboxylic acid group of the polyelectrolyte through
the well-known carbodiimide chemistry.
Figure 3.2
Functionalization of Au NPs with biotin-amine.
When compared to Au NPs, Ag NPs are more unstable, and their
bioconjugation is not as straightforward, limiting their application in
DNA assays. One way to overcome this limitation is the preparation of
Ag NPs in the presence of bifunctional chemicals such as hydrazone-
functionalized (pyridyldithiopropionyl) dextran.
61
The hydrazone-
functionalized (pyridyldithiopropionyl) dextran then reacted with
an excess of oligonucleotide that terminates in an aromatic aldehyde
group.
















Search WWH ::

Custom Search