Biomedical Engineering Reference
In-Depth Information
approximation for the particular wavelength used to excite the NP.
At the other end of the dimensional scale, diminishing conductivity
of the metal NPs as a result of electronic scattering processes at the
particle's surface will reduce the quality of the LSPR and also the
SERS enhancement.
10
11.1.3 Chemical Enhancement
E
vac
ǻ
E
i
w
LUMO
HOMO
Metal
Adsorbate
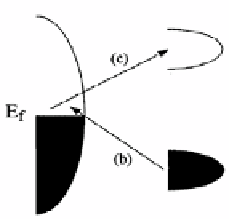
Figure 11.3
Energy level diagram for a molecule adsorbed on a metal
surface. The occupied (HOMO) and unoccupied (LUMO)
molecular orbitals are broadened into resonances by their
interaction with the metal states; orbital occupancy is
determined by the Fermi energy. The molecule-to-metal
charge transition is indicated by the black arrows. Work
function (
w
) of the metal and ionization potential (
Δ
E
i
)
of adsorbate molecules are depicted with blue arrows.
Reproduced with permission from Ref. 30 with permission.
See also Color Insert.
Another contribution to the observed SERS intensity is generally
referred to as “chemical enhancement.”
5
This usually applies to
the adsorbates strongly bond (chemisorbed) to the metal surface
so that the Raman scatterer is essentially an adsorbate-surface
complex, analogous to a metal-ligand or coordination complex.
The creation of the surface complex not only increases the Raman
scattering cross-section, it also leads to resonances in the visible
region of the spectrum due to a metal-to-molecule or molecule-to-
metal transitions. Figure 11.3 describe this process in a pictorial
form. In many molecules, the Fermi energy of the noble metal



















Search WWH ::

Custom Search