Biomedical Engineering Reference
In-Depth Information
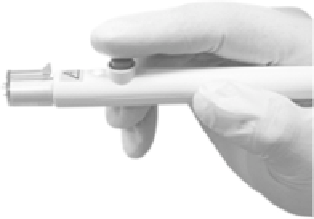
FIGURE 14.4
CELLECTRA
®
-ID. electroporation. device.. CELLECTRA
®
-ID. is. used. for. intradermal. injections,. contains. three.
26-gauge.needle-electrodes.to.generate.the.electric.ield.(0.2.Amp.constant.current),.and.penetrates.3.mm.into.
the.site.of.injection.
factors,. including. depth. of. needle-electrode. penetration.. Intramuscular. DNA. injections.
will.require.needle-electrodes.to.conduct.voltage.and.create.an.electrical.ield.around.the.
site. of. DNA. injection.. One. potential. solution. for. this. problem. is. to. decrease. the. area. of.
DNA.transfection..Although.the.intensity.of.the.electric.ield.must.be.maintained,.decreas-
ing.the.area.of.transfection.would.reduce.the.voltage.and,.consequently,.overall.pain..In.
this. regard,. improved. manufacturing. methods,. which. have. allowed. for. the. production.
of.more.concentrated.vaccines,.have.been.especially.important.
50
.The.more.concentrated.
DNA.solution.will.ostensibly.decrease.the.total.volume.of.injected.vaccine,.and.therefore.
decrease.the.area.of.needle.insertion.without.altering.the.eficiency.of.DNA.transfection.
14.5.3 A Fundamental Change in DNA Vaccines: Electroporation
and Vaccine Development Go Hand in Hand
Recent.advances.of.DNA.delivery.via.electroporation.have.illuminated.a.signiicant.rela-
tionship. between. DNA. vaccines. and. electroporation.. While. these. two. ields. have. been.
traditionally.distinct,.the.development.of.future.vaccines.will.entail.careful.consideration.
of.both.aspects,.and.therefore,.these.two.ields.should.not.be.considered.mutually.exclu-
sive.. For. example,. some. pathogens,. such. as. inluenza,. respiratory. syncytial. virus. (RSV).
and. HBV,. may. require. primarily. an. antibody. response. to. prevent. infection,. thus. favor-
ing.intradermal.delivery.of.vaccines..Conversely,.other.pathogens,.such.as.HCV,.require.
a.strong.T.cell.response,.thus.favoring.intramuscular.delivery.of.vaccines..Therefore,.the.
development.of.speciic.vaccines.must.be.coupled.with.not.only.the.design.of.DNA.plas-
mids,.but.also.the.choice.of.the.electroporation.system.
Inovio. currently. uses. two. different. electroporation. applicators.. The. CELLECTRA
®
.
Adaptive.Constant.Current.Electroporation.Device.is.currently.in.use.in.phase.I.clinical.
trials.to.deliver.HPV.and.HIV.DNA.vaccines..It.consists.of.three.components:.(1).a.pulse.
generator.that.is.enclosed.in.a.water-resistant.case.for.housing.the.circuitry.and.software,.
(2).an.ergonomic.applicator.that.is.connected.to.the.electroporation.system.unit.with.an.
attached. power. cord,. and. (3). a. sterile. disposable. array. with. the. needle-electrodes. and.
an. injection. port. to. deliver. the. plasmids.. One. of. the. CELLECTRA
®
. devices. is. designed.
for.intramuscular.injections.and.contains.ive.21-gauge.needle-electrodes.to.generate.the.
electric. ield. (0.5. amps. constant. current). that. will. penetrate. between. 1. and. 2. cm. at. the.
site.of.injection..The.other.device,.CELLECTRA
®
-ID,.which.is.newer.and.used.for.intra-
dermal.injections,.employs.a.pulse.generator.identical.to.that.of.the.intramuscular.version,.