Biomedical Engineering Reference
In-Depth Information
The second type of force that comprises adhesive forces is electrostatic forces. These can
arise from either excess charge on a molecule or an electrical double layer. The force asso-
ciated with charge should be familiar from a previous classical physics class and is
described by Coulomb's law, which states that
1
q
1
q
2
r
2
F
5
ð
7
:
15
Þ
4
πε
0
where
q
is the excess charge on molecule 1 and 2, respectively;
r
is the distance between
the two molecules; and
ε
0
is the permittivity of free space. These charges can be removed if
the substances are “grounded” or more likely the charges can be transferred to another
molecule. The electrical double layer is more of a concern in biological applications, where
you encounter the interaction of a small particle (that may or may not have a charge associ-
ated with it) with a larger particle with a surface charge that acts as a boundary. For biolog-
ical applications, this can be considered as the intercellular cleft (or a protein channel), in
which small potentially charged molecules will permeate. The charge on the boundary
attracts opposite charges within the solution. As an example, if the surface has a partial
positive charge, then the partial negative charge present on the oxygen atom within water
will be attracted to the surface (
Figure 7.4
; we use the example of water because this is the
most likely bathing medium in the body). This suggests that a second, potentially less
strong, partial positive layer will be formed from the aligned hydrogen atoms that compose
water. A second layer of water will form on top of this original layer, until the electric field
generated from the boundary weakens to a point that it can no longer maintain this orga-
nized charge structure. The surface potential on the boundary is termed the Nernst poten-
tial and the potential at the point that the layer begins to breakup is termed the Stern
potential. The entire distance between the boundary and the point where the charges in
solution break up is called the Stern layer. The Stern layer is characterized by highly orga-
nized solutes, and in biological applications this is typically water. Directly next to the Stern
layer there is a slight organization of the charged molecules until the shearing plane, where
the organization resembles a solution in contact with an uncharged surface and is therefore
organized randomly. In the second layer, water is partially organized and partially random.
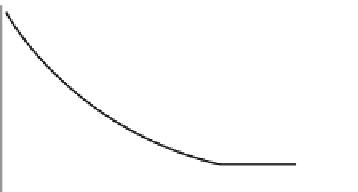
FIGURE 7.4
Charged
surface
Electrostatic forces near a
charged surface, which tend to organize water
(or other ions) close to the boundary. The layer
that is closest to the charged boundary and has
the highest organization is termed the Stern
layer. A second layer, of mostly organized
charged species, is termed the electric double
layer. Beyond the electric double layer,
--
--
--
--
++
+
+
+
--
-
+
+
+
-
+
+
-
-
-
+
Nernst
potential
the
Stern
potential
charged species would resemble
the bulk
properties.
Zeta
potential
Stern
layer
Electric double
layer
Distance from the
charged surface












Search WWH ::

Custom Search