Biology Reference
In-Depth Information
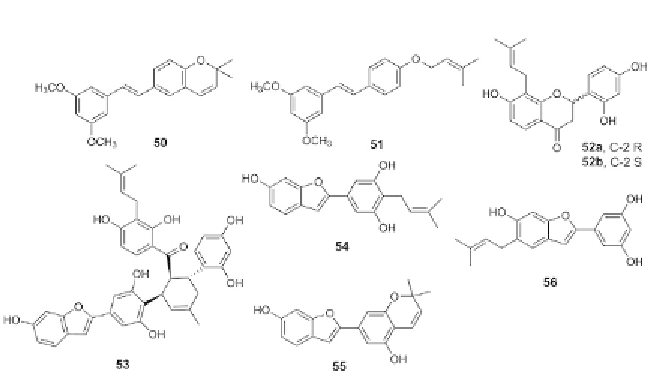
Figure 3.7 Chemical structures for stilbenes, compounds 50-56.
2.6. Stilbenes
(Fig. 3.7)
Two stilbenes, lonchocarpene (
) and 3,5-dimethoxy-4
0
-
O
-prenyl-
trans
-
50
stilbene (
), were isolated from
Deguelia rufescens
, and both showed a potent
concentration-dependent inhibition activity on
a
-glucosidase from bacteria
bene, a new group of
a
-glucosidase inhibitors apart from iminosugars
Among the isolated prenylated stilbenes, (2
R
)/(2
S
)-euchrenone a
7
(
52a
,
52b
51
)
,
chalcomoracin (
53
), moracin C (
54
), moracin D (
55
), and moracin
N(
) exhibited potent inhibition activities on
a
-glucosidase from yeast
with IC
50
values of 6.28, 2.59, 4.04, 2.54, and 2.76
m
M.
To summarize, there are plenty of reports on natural products with
a
-glucosidase inhibition activity; however, most of them are limited to
measuring the IC
50
values using yeast
a
-glucosidase and apply pNPG as sub-
strate, which is not a natural substrate for the enzyme. In addition, since dif-
ferent labs may use slightly different conditions, it is difficult to compare the
IC
50
values obtained from different labs. As such thus it leaves plenty of
uncertainty before these compounds can be utilized as functional food ingre-
dients for human consumption. Measurement of the inhibitor activity shall
be done using mammal
a
-glucosidase. The performance of these
compounds in a real food system shall be evaluated because the potential
interaction of these compounds, particularly polyphenolic compounds,
can compromise their activity against
a
-glucosidase. Acarbose is not
well received by patients because of its side effects that include flatulent,
56