Biology Reference
In-Depth Information
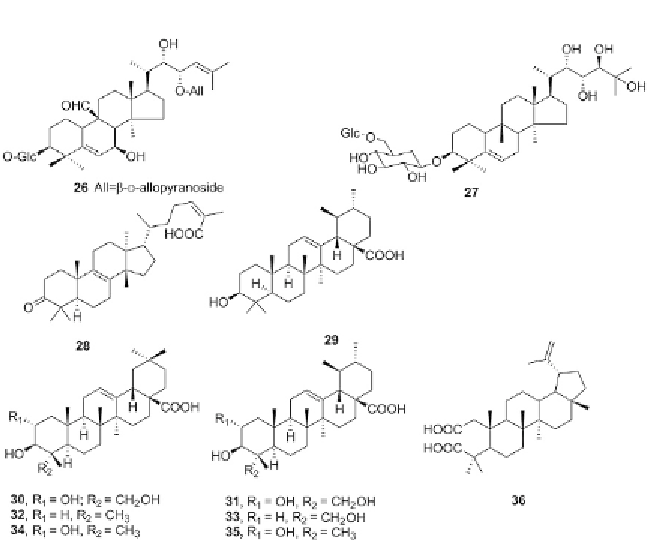
Figure 3.4 Chemical structures for terpenoids, compounds 26-36.
momordicoside M (
) isolated from the fruits of
M. charantia
were reported to possess a moderate
a
-glucosidase inhibitory
activity with a 18.63% and 21.71% inhibition at the concentration of
anism remain to be characterized.
Some triterpene acids such as pistagremic acid (
26
) and momordicoside A (
27
) isolated from dried
galls extract of
Pistacia integerrima
were reported to possess a potent enzyme
inhibitory activity both against yeast and rat intestinal
a
-glucosidase with
acarbose was found to be selective and 12 times more potent against rat intes-
tinal
a
-glucosidase (IC
50
, 62.74
m
M), as compared to the yeast a (IC
50
,
780.21
m
M). The molecular docking simulations revealed that the binding
cavity of yeast enzyme seems to be slightly narrow as compared to the bind-
ing pocket of the mammalian enzyme. The difference in size of the binding
cavities of both enzymes affected the inhibitory activity of the acarbose.
While the molecular shape and size of
28
made it capable of easily penetrat-
ing the binding pockets of both enzymes. Other triterpene acids such as
ursolic acid (
28
29
) isolated from the aerial parts of
Pimpinella candolleana
also