Biomedical Engineering Reference
In-Depth Information
Graham et al. 1999). These variances in temperature sensitivities
do not make MT a readily reliable method for MRTI.
temperature sensitivity coefficient of the water PRF is relatively
the same for different tissues and is close to pure water, which
has a temperature sensitivity coefficient (TSC) of -0.010 ppm/ºC
(Hindman 1966; Figure 3.3). Many studies have been performed
both
ex vivo
and
in vivo
to measure the temperature sensitiv-
ity of the PRF. McDannold presented a thorough review of the
TSC of the PRF using both complex phase-difference (CPD) and
chemical shift imaging (CSI) techniques. Most TSC values in tis-
sues have been found to be between -0.009 and -0.010 ppm/°C
(McDannold 2005). However, some
in vivo
studies have measured
values above and below this range. The difficulty of accurately
measuring the temperature sensitivity coefficient
in vivo
remains
a challenge that needs to be addressed. In practical implementa-
tion, many investigators measure tissue sensitivity
ex vivo
or use
literature-obtained values. These values tend to be near -0.01
ppm/°C for a wide variety of tissue.
A source of error in the PRF technique arises when there is a shift
in the local magnetic field unrelated to the temperature-dependent
chemical shift. One instance in which this can happen is a tem-
perature-dependent change in the bulk magnetic susceptibility
(De Poorter 1995; Peters et al. 1999), which can also be affected by
the orientation of the heat source in relation to the magnetic field.
Additionally, the temperature-dependent electrical conductivity of
tissue can induce temperature-dependent phase errors when large
amounts of tissue are heated (Peters et al. 2000). While this effect is
unlikely to be insignificant for ablation of small volumes of tissue,
the impact may become significant when large volumes of tissue
are heated, such as with hyperthermia. In these cases, a multi-echo
sequence is recommended to offset this shift.
In fatty tissue, De Poorter et al. measured an overall
TSC of -0.0097 ppm/°C with a susceptibility constant of
-0.0013 ppm/°C, giving a corrected field shift of -0.0088
ppm/°C (De Poorter 1995). In a separate study by a different
investigator using lipid (bulk methylene) as an internal refer-
ence, the TSC of the difference was measured and found to be
around -0.00852 ppm/°C (Kuroda 2005). A study using a line
3.3.3 temperature Dependence of the Water
proton resonance Frequency (prF)
Temperature sensitivity of the water PRF was first reported
by a group led by Pople in 1958 (Schneider 1958) and later by
Hindman (1966). This dependence is due to the relatively weak
hydrogen bonding between hydrogen protons and oxygen nuclei
of water. When temperature increases, the increased kinetic
energy of the water protons results in a longer hydrogen bond
with other water molecules and a shorter covalent bond between
the hydrogen and parent oxygen nuclei. This results in the pro-
ton lying in closer proximity to the electron cloud of oxygen,
thereby changing the proton chemical shift, σ (Figure 3.2). The
PRF (
f
) can therefore be expressed as a function of the chemical
shift where
f
=γ
B
(1
−σ
),
(3.10)
0
γ is the gyromagnetic ratio (42.58 MHz/Tesla for hydrogen
atoms), and
B
0
is the applied magnetic flux density. The shielding
constant, σ, theoretically is related to the magnetic field associ-
ated with the electronic structure around the nucleus. Usually
empirical approaches are used to describe the shielding constant
since theoretical calculation requires extensive knowledge of the
electron density in the ground and excited states of the molecule
as well as the excitation energies. Generally, this chemical shift
caused by the shielding constant is the contribution of the elec-
trons surrounding the nucleus as well as other surrounding mol-
ecules around the nucleus (Haacke et al. 1999).
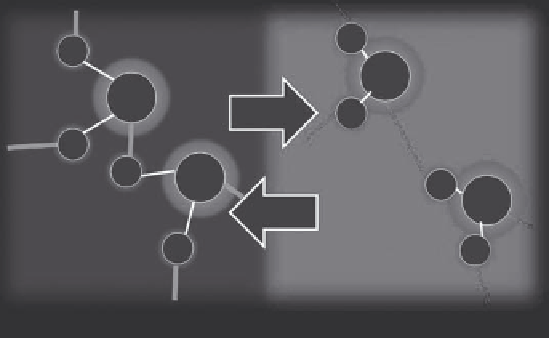
Measuring the PRF shift as a function of temperature or mea-
suring the distances between two spectral peaks to measure tem-
perature has long been used in NMR in the field of analytical
chemistry. When compared to other MRTI-based parameters, the
H
H
O
O
T
H
H
H
O
H
O
T
H
H
f
=
γ
B
0
(1 -
σ
(
T
))
f
=
γ
B
0
(1 -
σ
(
T
+
∆
T
))
FIGURE 3.2
The proton resonance frequency phenomenon.
As temperature increases, hydrogen bonds lengthen (gray) and covalent bonds
(black) pull the proton (H) closer to the parent oxygen (O). The proton experiences a downfield shift in its Larmor resonance frequency (
f
) due to
increased shielding (Δσ) from the electron cloud.