Biomedical Engineering Reference
In-Depth Information
as MR thermometry will be necessary to document the degree
of the change at the periphery, the gradient that must exist from
the center of the injection outward, and the time course of the
decay back to baseline.
Given that the peak temperature starts to decline as soon as
the input stops (analogous to running out of fuel), and that it is
almost certainly not as hot at the edge as measured near the tip,
other mechanisms must also be active. Two likely candidates are
the hyperosmolarity of the reaction products and Hofmeister
effects. To understand osmolarity better, it is helpful to have some
context within the human body. Most cells live in a milieu in the
range of 270-300 mOsm. The exception is in the distal convo-
luted tubule of the kidney, where an environment of up to 1200
mOsm has been observed in a normal kidney as it concentrates
urine. This is an adapted, chronic condition at body temperature.
In contrast, with a 15 M acid and base, the osmotic strength of the
salt solution would be 15 kOsm. This approaches two orders of
magnitude higher in concentration than almost anywhere in the
body other than the specialized area in the kidney noted previ-
ously, and in an ablation it is an abrupt change. Furthermore, to
varying degrees, the change in temperature will likely have some
effects that are cooperative. A final note on this topic is that in
a number of reagent choices, it is entirely possible to generate a
salt at a concentration much higher than the solubility even at
elevated temperatures. Thus, in the local environment it would be
possible in theory to essentially inject a salt crystal deposit.
A detailed explanation of Hofmeister effects is outside the
scope of this discussion, but a minimal understanding may shed
some additional light on another aspect of how thermochemical
ablation may work. Briefly, salts vary in their ability to either
stabilize or destabilize (i.e., denature) proteins.
36-38
The eminent
protein chemist Franz Hofmeister late in the nineteenth cen-
tury formalized this in the series that now bears his name and is
shown in Figure 19.15.
NH
4
+
K
+
Na
+
Li
+
Mg
++
Ca
++
Guanidinium
+
SO
4
2-
HPO
4
2-
OAc
-
Citrate
-
Cl
-
NO
3
-
ClO
3
-
I
-
ClO
4
-
SCN
-
Kosmotropic/ordered
Chaotropic/denaturing
FIGURE 19.15
The Hofmeister series of anions and cations arranged
according to denaturing ability. The more chaotropic (denaturing, from
chaos or disorder) species are on the right side of the spectrum, and the
more kosmotropic (favoring order) species are on the left.
He ranked various salts according to their effects on proteins
and noted that anions generally have more effect than cations.
It is useful to note where in the Hofmeister series the current
salt, NaOAc, is positioned. As is apparent in the figure, this salt
is at best intermediate in its intrinsic ability to denature proteins.
Theoretically, then, the choice of the salt could be altered more
in the direction of a denaturant. Few things are as simple as they
seem, however, and thermochemical ablation using acid-base
chemistry definitely entails other considerations. In an effort to
shed more light on this, we studied the effects of exposure to
numerous salts at 37°C at various concentrations using the MTT
viability assay. This allowed us to separate the effects of salt and
concentration from a temperature excursion; an example assay
is shown in Figure 19.16 with sodium acetate as the salt. We used
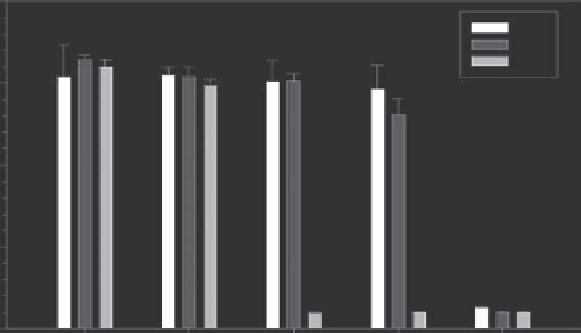
HuH7 cells as they represent a human hepatoma lineage.
Although little toxicity is observed at six hours with 200-400
mM sodium acetate, it is clear that few cells survive an extended
exposure at this concentration. How much of this result is simply
a hyperosmolarity effect rather than anything more complex is
yet unresolved.
From the preceding discussion, we must keep in mind which
reagents would be used to generate the salts, and determine how
much of an energy release could be expected. This is predicated
0.8
2 h
6 h
24 h
0.6
0.4
0.2
0.0
0
100
200
400
800
Sodium acetate (mM)
FIGURE 19.16
MTT viability assays of sodium acetate exposure on HuH7 cells with respect to duration and concentration. The survival drops
dramatically at 24 h of exposure even at lower concentrations. These conditions might be observed at the periphery of a treated area, and the con-
centrations would be much higher closer to the center.