Biomedical Engineering Reference
In-Depth Information
TABLE 19.1
Broad Categories of Exothermic Chemical
Transformations That Could Be Exploited for Ablation of
Tissues
Sources of Chemical Energy
Acid-base neutralization (formation of water)
Hydrolysis
Oxidation-reduction reactions (redox chemistry)
Heat of solvation
Ring strain energy
TABLE 19.2
Criteria for Selection of Reagents Potentially
Useful in Thermochemical Ablation
Some Selection Criteria
Liquid to facilitate injection
Easy to handle
Safe to handle
Low cost
Readily available
Adequate release of energy
useful range to release enough energy, and that the salts pro-
duced are safe at the doses necessary, it should be possible to
coagulate tissues by this method. Details about precise reagent
choice and regarding delivery therefore need to be investigated
and addressed. Chemistry will be discussed in more detail first,
followed by device development and applications.
In order to assess a number of potential combinations, devel-
opment of a phantom was necessary. Criteria for a suitable phan-
tom were identified and are listed in Table 19.3.
Ultimately, a commonly available household product called
baby oil gel found in pharmacies and large discount stores was
drafted into service as a simple calorimeter. Using this approach,
we characterized the relationships between reagent strength
and concentration.
32
The chemistry was remarkably consistent
under these conditions, showing clearly that for a given con-
centration, the stronger reagents evolved larger amounts of heat
energy. It was also clear that higher concentrations of reagents
released more energy.
33
This makes it possible to compare a wide
variety of reagents and conditions.
Much attention is given to the ability to monitor treatment
progression in procedures such as ablations. With chemical
ablation, some have added iodinated contrast material to the
reagent, such as was reported using acetic acid doped with a
small amount of contrast.
34
The difficulty with having separate
imaging and therapeutic agents is that it is based on the assump-
tion that the materials interact with and spread through tissues
equally. A more rigorous approach would consider the hetero-
geneous nature of the tissues to be analogous to the packing
material in column chromatography. In such a case, the injec-
tion point would be considered to correspond to the top of a
column, and the materials in the mixture would be expected to
pass through at different rates. It would be no surprise to find
O
O
+ Heat + HCI
CI
Nu:
OH
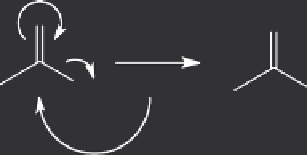
FIGURE 19.3
Hydrolysis of acetyl chloride by water. This reactive
compound is converted to acetic acid in a vigorous reaction that simul-
taneously generates an equivalent of an even stronger acid, hydrochlo-
ric acid.
OH
O
+ Heat
Nu
Nu:
FIGURE 19.4
The ring opening of a compound with strain energy
such as an epoxide can produce an exotherm that is extremely energetic
(127 kJ/Mole).
Opening the ring of an epoxide in a reaction with a nucleophilic
species evolves approximately 127 kJ/mole, shown in Figure 19.4.
This may be attractive from one point of view, but these com-
pounds are typically alkylating agents and therefore many are
carcinogenic.
19.1 practical Considerations
There are several constraints to bear in mind for exploiting any
chemistry for a percutaneous therapeutic application. Several
criteria are listed in Table 19.2.
In general, liquids are preferable to solids because of the ease
of injection into tissues and the ability to spread from the injec-
tion site. The reagents should be easy and safe to handle, with
minimal or very low risk to both patient and personnel should
any spillage occur. Low cost and ready availability both would
be desirable, and the amount of energy released would need to
be commensurate with the task at hand. Applying these crite-
ria as filters for the categories discussed herein leads naturally
to acid-base neutralization. Provided the pK
a
values are in a
TABLE 19.3
Criteria for a Phantom (Calorimeter) for
In Vitro
S
tudies of Candidate Reagents for Thermochemical Ablation
Requirements for a Useful Phantom
Clear
Colorless
Medium viscosity
Neutral density
Nontoxic
Low cost
Chemically stable to conditions
Nonvolatile