Biomedical Engineering Reference
In-Depth Information
and
z
is the depth. Direct illumination is easy to apply but is
limited by the penetration depth of the laser.
For deep tissue, interstitial laser fibers can be used, for example,
to treat brain tumors (Schwartz 2009). The laser attenuates more
rapidly than the case given by Equation 18.4 because of the radial
geometry. Usually a water-cooled applicator is applied to reduce
the tissue temperature near the fiber and avoid vaporization and
carbonization of the tissue. Endoscopes and catheters are typi-
cally used for vascular disease, for example, in cardiology.
In summary, for tumor photothermal therapy, the main
methods of applying laser are either direct illumination for
surface tumors or minimally invasive interstitial fiber for deep
tumors. To more quantitatively obtain the laser fluence in the
tissue with or without the presence of GNPs, light transport
models are needed and reviewed next.
10
15
Photodisruption
10
12
Photoablation
Plasma-
induced
ablation
10
9
10
6
10
3
ermal interaction
10
0
Photochemical interaction
10
-3
18.3 Laser Fluence Estimation
in tissues
10
-15
10
-12
10
-9
Exposure time (s)
10
-6
10
-3
10
0
10
3
(a)
The introduction of NPs alters the optical properties of the tissue
and hence the thermal response. In this section, the methodolo-
gies commonly used in modeling the light transport and recent
applications for NP laden tissue are reviewed. The light transport
is described by the radiative transport equation (RTE), given by
(Welch 1995, Modest 2003):
10
15
ermal therapy
Bubble formation
Plasma generation
10
12
10
9
(,
ˆ
)
dL rs
ds
∫
10
6
(,
ˆ
)
( ,
ˆ
)
(
ˆ
,
ˆ
)(,
ˆ
)
( ,
ˆ
)
=−µ
Lrs
−µ
Lrs
+µ
pssLrsdSrs
′
ω+
a
s
s
Reduced exposure time
π
10
3
(18. 5)
(,
ˆ
)
is the radiance in position
r
and
ˆ
direction with unit
[W/m
2
/sr] and the integration of
Lrs
where
Lrs
10
0
(,
ˆ
)
over all directions is the
fluence (
I
),
µ
is the absorption coefficient [1/m],
µ
is the scattering
coefficient,
pss
Reduced laser irradiance
1 kJ/cm
2
10
-3
1 mJ/cm
2
1 J/cm
2
(
ˆ
,
ˆ
′
is the scattering phase function [1/sr] describing
the contribution of scattering from
ˆ
′
direction to
ˆ
direction, and
Srs
10
-15
10
-12
10
-9
Exposure time (s)
10
-6
10
-3
10
0
10
3
(,
ˆ
)
is the source term. It states that the change of light fluence
is determined by losses from the absorption (first term) and scat-
tering (second term), and gain from scattering (third term) and
energy source (last term). RTE is an integro-differential equation
and is difficult to solve directly. Several approximations for solv-
ing the RTE equation have been used for laser GNP heating and
in some cases can be compared directly to experimental results.
By employing the P1 approximation (first approximation
to spherical harmonics) to RTE, Bayazitoglu and coworkers
(Tjahjono 2008, Vera 2009) computationally studied the effect of
NP scattering and absorption on SAR. It was shown that GNPs
with high scattering (for example, gold nanoshell with high
C
sca
/
C
ext
in Table 18.2) increase the internal diffuse radiation and cre-
ate a more even SAR, while GNPs with high absorption produce
a large amount of SAR at the entry region (i.e., the region near
the laser source). However, this approach is difficult to apply for
complex geometries.
Another simplifying assumption to solve RTE is the diffusion
approximation (DA), which is applicable for highly scattering
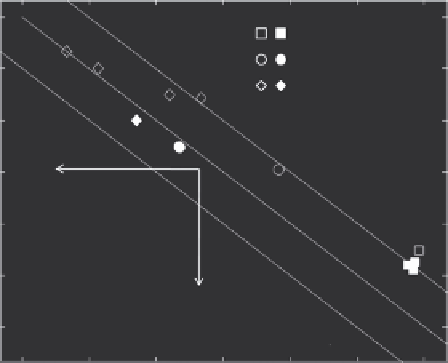
(b)
FIGURE 18.2
(a) Map of laser-tissue interactions including pho-
tochemical, photothermal, photo ablative, plasma induced ablative,
and photodisrutptive modes. Note that this map shows the approxi-
mate position of the interaction modes. (Reproduced from Niemz, M.,
Laser-Tissue Interactions: Fundamentals and Applications
, Springer
Verlag, 2004. With kind permission from Springer Science +Business
Media B.V.) (b) The thresholds of laser parameters for thermal therapy,
bubble formation (photo ablative), and plasma generation. Open sym-
bols are without GNP, and filled symbols are with GNP. The dashed
lines in the figure indicate the same amount of energy input in terms of
J/cm
2
. (From Müller, G. and Roggan, A., Laser-induced interstitial ther-
motherapy. SPIE-International Society for Optical Engineering, 1995;
Hirsch, L. et al.,
Proceedings of the National Academy of Sciences of the
United States of America,
100, 2003; Gobin, A. et al.,
Nano Lett,
7, 2007;
von Maltzahn et al.,
Cancer Res,
69, 2009; Vogel, A. and Venugopalan,
V. ,
Chem. Rev,
103, 2003; Takeda, Y., Kondow, T. and Mafuné, F.,
J. Phys.
Chem. B,
110, 2006.)