Biomedical Engineering Reference
In-Depth Information
(a)
40
Blood
Without magnet array
35
Heart
30
Lung
25
Liver
20
Spleen
15
Kidney
10
Tumor
5
Brain
0
5 min
15 min 30 min 1 h
Time after MF application
15.5 h
24 h
52 h
114 h
(b)
40
With magnet array, 50 mT
35
30
25
20
15
10
5
0
5 min
15 min
30 min 1 h
Time after MF application
15.5 h
24 h
52 h
114 h
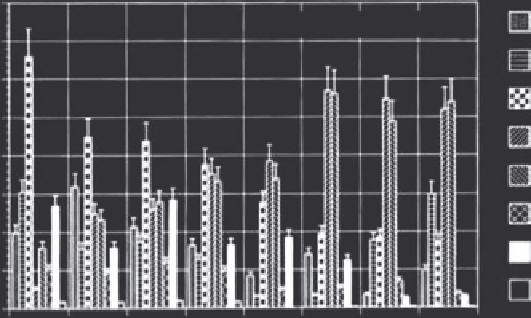
FIGURE 17.10
Tissue iron content of selected organs of C3H tumor bearing mice after intratumoral magnetic fluid administration without (a)
and with (b) an external magnet array. (From Jordan, A. et al.,
International Journal of Hyperthermia
13, 6, 1997.)
to increase retention in the tumor. The resulting time-dependent
biodistribution is included in Figure 17.10. Retention in the tumor
was increased significantly through the use of the magnetic array,
as well as observed decreases in iron deposition for bystander
organs. Intratumoral steadystate temperatures of 47 ± 1°C were
maintained for 30 minutes with a whole body field at 520 kHz and
6 to 12.5 kA/m. No significant heating was observed in other tis-
sues. The histological results were fairly heterogeneous and likely
reflected inhomogeneity of nanoparticle deposition. Some of the
tumors showed no evidence of regrowth after 50 days, while others
grew readily after treatment.
Magnetic fluid hyperthermia was also used for treatment
in rat tumor models with glioblastoma multiforme and pros-
tate carcinoma (Jordan et al. 2006; Johannsen et al. 2005).
Intratumoral injection of aminosilane-coated particles produced
stable nanoparticle deposits, capable of multiple treatments under
alternating magnetic fields variable from 0 to 18 kA/m at 100
kHz. Intratumoral temperatures in the glioblastoma model were
measured at 43°C to 47°C (held for 30 minutes), and resulted
in an increased survival rate of 1.7- to 4.5-fold over the control.
Maximum intratumoral temperatures of up to 70°C were mea-
sured in the prostate carcinoma model, with mean maximum
and mean minimum temperatures of 54.8°C and 41.2°C, respec-
tively. Treatment resulted in a 44% to 51% inhibition of tumor
growth over the control. Post-mortem histological analysis
showed mean iron biodistribution at 82.5% in the tumor, 5.3%
in the liver, 1.0% in the lung, and 0.5% in the spleen.
An additional
in vivo
study demonstrated the potential for
magnetic fluid hyperthermia as a combinatorial therapy, with
adjunct radiotherapy treatment in a rat tumor model with
prostate carcinoma (Johannsen et al. 2006). Aminosilane-coated
nanoparticles were injected intratumorally, with two subse-
quent thermal treatments or two radiation doses ranging from
2 × 10 Gy to 2 × 30 Gy. Thermal therapy was also combined
with the lowest radiation dosage. Mean maximum and mean
minimum intratumoral temperatures were measured at 57.1°C
and 42.5°C, respectively. The combined low-dose radiation
thermal treatment matched the effectiveness of the high-dose
radiation therapies, with a reduction in tumor growth of 87.5%
to 89.2% over controls.