Biomedical Engineering Reference
In-Depth Information
50
(d)
(a)
45
40
35
30
25
20
15
10
5
0
LTSL during HT
LTSL before HT
LTSL during HT
LTSL split dose
(b)
0
10
20
30
Time (min)
40
50
(e)
HT
HT
dose
1.0
Control
0.9
0.8
0.7
0.6
0.5
0.4
0.3
LTSL before HT
HT
Free Dox
Free Dox + HT
(c)
Dox/Mn-LTSL alone
Dox/Mn-LTSL before HT
Dox/Mn-LTSL during HT
Dox/Mn-LTSL split dose
0.2
0.0
0.1
LTSL before and
during HT
0
20
40
Time (days)
60
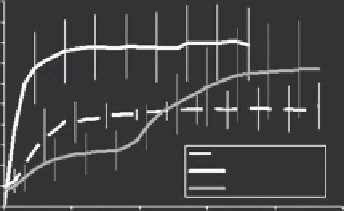
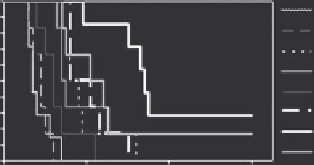
FIGURE 16.7
Intratumoral drug deposition patterns for three sequences of HT with LTSL-MnDox, as assessed using T1-based MRI. (a) Drug
delivery pattern when tumor is heated before and during liposomes administration. (b) Drug delivery pattern when drug is given prior to heating.
(c) Drug delivery pattern is more uniform if half is given prior to heating and the other half after heating has started. (d) Drug concentration in
tumor over time, as measured using MRI. More drug is delivered if the tumor is heated first. The highest overall drug concentration and antitumor
effect (e) was observed for the sequence that used HT first, followed by LTSL-Dox (a). (Reproduced from Ponce, A. M. et al.,
Journal of the National
Cancer Institute
, 99, 2007. With permission.)
provided previously by Viglianti, she was able to measure drug
concentration distributions in these tumors, based on the change
in T1 relaxation in each voxel of the tumor. The heating device
consisted of a catheter placed centrally along the axis of the
tumor, through which hot water was circulated. This device was
MR compatible, which permitted visualization of drug deposi-
tion in real-time during heating. Three scenarios were followed,
which altered the drug deposition pattern: (1) If the tumor was
preheated before drug was administered, the drug deposition
pattern was primarily peripheral. This occurred because the tem-
perature of the periphery was above the transition temperature
for the liposome and the inflow of blood to the tumor started at
the periphery (Figure 16.7). (2) If the liposomes were adminis-
tered first, followed by the onset of heating, the drug deposition
pattern was centrally located and spread outward as heat spread
radially from the heating catheter. (3) A more uniform drug
delivery pattern was achieved by delivering half the dose and
starting to heat, followed by injection of the second half of the
dose after the tumor achieved thermal steady state. Interestingly,
the greatest antitumor effect was observed for case #1. The reason
for the greater antitumor effect was surmised to be the result of
an antivascular effect, where the greatest concentration of drug
was deposited in the region of the feeding tumor vasculature.
Investigators examining high intensity focused ultrasound
have followed the lead of Ponce to publish other papers using
this same type of liposome, in combination with HIFU, to
dose paint drug into target tumors (Mylonopoulou et al. 2010,
Negussie et al. 2011, Staruch et al. 2011).
16.8 Clinical applications of LtSL-Dox
16.8.1 Canine trial
The first phase I study of LTSL-Dox was conducted by Hauck
et al. in dogs with spontaneous canine tumors (Hauck et al.
2006). Privately owned dogs with solid sarcomas or carcino-
mas were enrolled. The tumors were required to be in a location
that was heatable using a 433 MHz microwave heating sys-
tem. Escalating doses of LTSL-Dox from 0.7 to 1.0 mg/kg were
administered at each of three courses, scheduled three weeks
apart. Pharmacokinetics were evaluated during the first treat-
ment cycle. A total of 21 patients were enrolled. The maximum
tolerated dose (MTD) was 0.93 mg/kg, which is approximately
10% lower than the reported MTD of free drug for the dog. The
first two dogs enrolled in this trial experienced anaphylactoid
reactions, characterized by a sudden transitory drop in blood
pressure, an increase in end inspiratory pressure, and in one
case extensive facial edema. Subsequent studies performed
in normal dogs identified this as being related to a profound
histaminemia. Subsequent animals were premedicated with
steroids and antihistamines, which minimized these toxici-
ties. These toxicities influenced the pretreatment regimens now
employed in human trials with this drug. The primary toxic-
ity encountered was neutropenia and renal toxicity (two with
fatal isothenuria), with dose-limiting toxicities (DLTs) observed
at 0.93 mg/kg. Grade 2 cardiac toxicities were observed at the
lower two dose levels.