Biomedical Engineering Reference
In-Depth Information
hyperthermia leads to relatively rapid vascular shutdown
(within 6 hr) that persists out to at least 24 hr after treat-
ment (Chen et al. 2004). The extent of vascular shutdown was
greater in the FaDu tumor line than in the 4T07 tumor, which
was found to be relatively resistant to the vascular damaging
effects of this formulation (Chen et al. 2008). Chen compared
the
in vitro
sensitivity of human umbilical vein endothelial
cells (HUVEC), 4T07 and FaDu, to doxorubicin, using a colo-
rimetric method. They concluded that the sensitivities of the
two tumor lines and HUVEC to LTSL-Dox were equivalent,
so the difference in antivascular effects could not be attributed
to variations in tumor or endothelial cell sensitivity (Chen
et al. 2008). However, it should be noted that the clonogenic
assay studies performed by Yarmolenko clearly demonstrated
that 4T07 is the most resistant of all tumor lines studied thus
far, including the FaDu tumor line (Yarmolenko et al. 2010).
Thus, one cannot rule out that variations in the vascular tar-
geting effects of LTSL-Dox are not attributable to variations in
tumor cellular sensitivity to this combination treatment. The
link between vascular sensitivity and cell sensitivity to killing
could be attributable to differences in VEGF levels, since this
pro-angiogenic cytokine, which is produced by tumor cells, is
known to promote survival of endothelial cells in response to
cytotoxic therapy (Gupta et al. 2002). If more tumor cells are
killed, VEGF levels could drop, thereby enhancing endothelial
cell sensitivity to this formulation. However, the issue is likely
more complex than this.
It has been reported recently that hyperthermia can increase
levels of the hypoxia-inducible transcription factor, HIF-1, in
tumors as a result of NADPH oxidase activation in tumor cells
(Moon et al. 2010). The increase in HIF-1 after hyperthermia
is associated with promotion of angiogenesis in 4T1 tumors.
Similarly, we have found that doxorubicin can increase HIF-1
levels in aerobic 4T1 tumor cells via a mechanism involving
reactive species formation (unpublished observations). Thus,
the difference in vascular sensitivity between tumor lines may
likely be a complex story involving the balance between how
many cells are killed versus whether or not HIF-1 and its down-
stream cytoprotective protein targets are upregulated in sur-
viving tumor cells. Clearly, more work is needed to probe this
question further, because in doing so, additional strategies may
be elucidated that could enhance the efficacy of the LTSL-Dox
formulation.
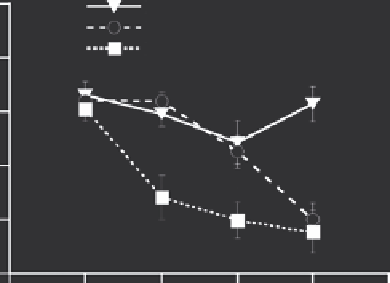
Chen went on to demonstrate that the antivascular effi-
cacy of LTSL-Dox with hyperthermia could be enhanced if
vascular permeability was enhanced using platelet activat-
ing factor (Figure
16.6) (Chen et al. 2008). The mechanism
underlying the enhanced vascular targeting effects of this
drug being associated with enhanced permeability has not
been elucidated, although the authors speculated that it
might be related to enhanced drug concentrations achieved
in vascular endothelium. One could also speculate that endo-
thelial cells that are subjected to platelet activating factor
may be more vulnerable to toxicity as a result of decreased
cell-to-cell contact.
10
LTSL-HT
LTSL-DOX-HT
PAF-LTSL-DOX-HT
8
6
4
*
*
*
2
0
-0.5
0
6
24
Time (h)
FIGURE 16.6
Skin-fold window chamber data showing changes in
microvascular density in 4T07 tumors treated with LTSL-Dox with
hyperthermia, empty LTSL with hyperthermia, and the combination of
platelet activating factor (PAF), LTSL-Dox and hyperthermia. *
p
< 0.05,
when comparing PAF + LTSL-Dox and hyperthermia to the other two
groups at 0 and 6 hr. At 24 hr, the extent of vascular shutdown between
empty LTSL and the other two groups was significant at 24 hr (
p
< 0.05).
Error bars - standard error of the mean (SEM). (From Chen, Q. et al.,
International Journal of Hyperthermia
, 24, 2008. With permission.)
16.7 Use of Imaging to Quantify
Drug Delivery from LtSL-Dox
and Establishment of treatment
Optimization Strategies
One of the methods used to load doxorubicin into liposomes
involves MnSO
4
. When this salt is loaded into the interior of a
liposome, doxorubicin enters into the liposome down its con-
centration gradient and is trapped in a complex with manganese
(Chiu et al. 2005). Viglianti utilized the paramagnetic proper-
ties of Mn
+ +
to evaluate Mn
+ +
and doxorubicin release from
LTSL-Dox-Mn, using MRI (Viglianti et al. 2004). He first dem-
onstrated that it was possible to measure content release
in vitro
,
because the relaxivity of the Mn
+ +
increases when it is released
from LTSL. This occurs because the interaction of Mn
+ +
with
bulk water increases, after release. The relaxivity of Mn
+ +
in bulk
water is also temperature dependent, so this needs to be kept
in mind when interpreting imaging results that incorporate this
type of contrast agent. In a subsequent paper, Viglianti demon-
strated that the extent of T1 shortening associated with Mn
+ +
release was linearly correlated with doxorubicin concentration
in tumors that were subjected to heating during MR acquisition
of T1 data (Viglianti et al. 2006).
Ponce evaluated how patterns of drug delivery by hyperther-
mia combined with LTSL-Dox-Mn could influence the efficacy
of this formulation (Ponce et al. 2007). The rapid drug release
properties of the LTSL provided a perfect foil for establishing a
rationale for drug dose painting as a means to enhance the antitu-
mor effects of LTSL-Dox. The method that she used involved real-
time imaging of Mn
+ +
delivery using MRI. Using the calibrations