Biomedical Engineering Reference
In-Depth Information
(a)
(b)
(c)
(d)
(e)
(f)
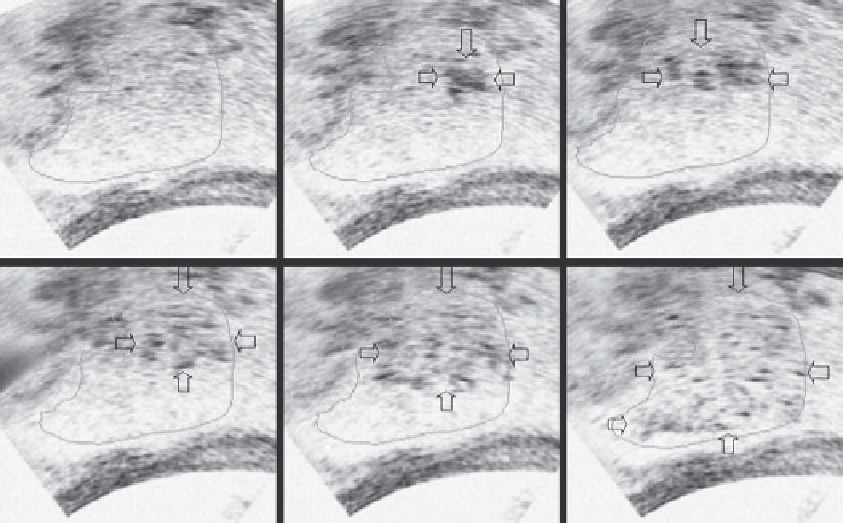
FIGURE 15.3
Grayscale change in big hepatocellular carcinoma obtained on real-time B-mode ultrasound imaging during HIFU procedure.
Compared to tumor imaging (outlined) before HIFU (a), hyperechogenic region (arrows) is seen in targeted region (b-e), until complete ablation
is achieved in this slice of the tumor (f).
after HIFU on the ultrasound image in treated tissues. Because
HIFU exposures consist of single exposures, multiple single
exposures, and scan track exposures, a cigar-shaped lesion and
a line-shaped lesion, as well as a slice-shaped lesion, can be obvi-
ously detected on real-time ultrasound imaging following rela-
tive HIFU exposures.
These grayscale changes observed during HIFU proce-
dures are of importance in the treatment of solid malig-
nancy. They provide feedback control imaging information,
monitor therapeutic effects, and control ultrasound energy
deposition in the focal volume during the ablation proce-
dure. Figure 15.3 shows the process of grayscale changes
on real-time ultrasound imaging during the ablation for a
patient with big hepatocellular carcinoma. While the focus
of HIFU is moved along one slice of the tumor in a scan-
ning fashion from deep to superficial part, a hyperechogenic
region is clearly detected on ultrasound imaging in this slice
of liver cancer.
Although the mechanism involved in tissue grayscale
changes during HIFU procedure is not clear, to date it is gener-
ally considered that gas bubbles that originate from acoustic
cavitation and tissue boiling (the vaporization of tissue water)
are the main factors. The development of ultrasound contrast
agents may improve the accuracy of ultrasound imaging for
evaluating the final therapeutic effect of vascularized tumor
immediately after the treatment is finished. To our knowledge,
however, there is no previous experience of real-time evalua-
tion of the therapeutic effects with ultrasound contrast agents
during HIFU procedures.
15.6.4 Follow-Up Imaging for
assessment of HIFU ablation
It is important to detect untreated regions of a targeted tumor
that require immediate retreatment, and to demonstrate com-
plete ablation of treated tumors as early as possible following
HIFU thermal ablation. Because postprocedural core biopsy
does not provide pathologic results for the entire treated tumor,
follow-up imaging is essential to evaluate the short-term efficacy
of
in situ
HIFU ablation. It is generally believed that, if untreated,
residual viable tumor foci will regrow, resulting in therapeutic
failure. The failure to detect focal areas of viable tumor in the
treated regions early after thermal ablation can lead to local
recurrence and to long distance metastases.
Prior to and following each treatment episode, contrast-
enhanced CT/MRI, ultrasonography, DSA, SPECT, and PET can
be employed to evaluate the response of targeted tumors after
HIFU procedure is finished. These modalities, anatomical or
physiological, can provide imaging information in determining
the therapeutic effects on both tumor vascularity and on cellular
functions immediately after HIFU, as well as changes in tumor
size during long-term follow-up periods.
Using color and power Doppler methods, ultrasonography can
assess changes in tumor vascularity after HIFU ablation. But, in
some cases, especially in the tumors with poor blood supply, ultra-
sound imaging is not sufficiently sensitive for detection of damage
to tumor blood vessels. Using ultrasound contrast agents, ultraso-
nography can provide imaging information to evaluate the thera-
peutic response of nonvascularized tumors after HIFU treatment.