Biomedical Engineering Reference
In-Depth Information
length and accommodate for dynamic changes in blood flow and
real-time heating pattern control. The angular or rotational heat-
ing pattern of these arrays can be controlled during fabrication by
scoring the transducer surface to isolate activate sectors (e.g., 90°,
180°, 270°, or 360°) to produce directional or angularly shaped
heating patterns. The orientation of these directional applicators
within an implant catheter can be used to target a treatment zone
while protecting critical normal tissue. Multi-applicator implants
of interstitial ultrasound applicators can be used to produce con-
tiguous zones of therapeutic temperatures [62,67] between appli-
cators with separation distances of 2-3 cm, while maintaining
protection in nontargeted areas. These catheter-cooled devices
suitable for percutaneous insertion have been evaluated with
transducer diameters between 1.2 mm and 1.5 mm and outer
catheter diameters between 2.1 mm (14 gauge) and 2.4 mm (13
gauge), respectively, with the latter being the most common con-
figuration [63,68].
Interstitial ultrasound hyperthermia integrated with HDR
brachytherapy for the treatment of locally advanced cervical
and prostate cancer has been performed using the 13 gauge
multi-transducer applicators [69] in a clinical pilot study. In
this setting, the hyperthermia is delivered either immediately
preceding or after the HDR radiation treatment using the same
catheters. Additional catheters are often used for temperature
measurement with multi-junction thermocouple probes to
monitor and provide treatment control. Applicator configura-
tions typically used for cervix and prostate treatment include
transducer diameters of 1.5 mm within 13 gauge catheter,
10-15 mm transducer lengths, 1-4 active transducer segments,
with 180° or 360° directional patterns. Given an implant pat-
tern typical of HDR brachytherapy for these sites, the length
of heating and directivity within each catheter is tailored
a
priori
to best fit the clinical target volume. A 3D optimization-
based treatment planning platform can determine best treat-
ment configuration plus the initial starting power levels to
each transducer segment [70]. The insertion depth and rota-
tion angle of the applicator within the plastic catheter can be
adjusted by sliding and rotating the device within the seal-
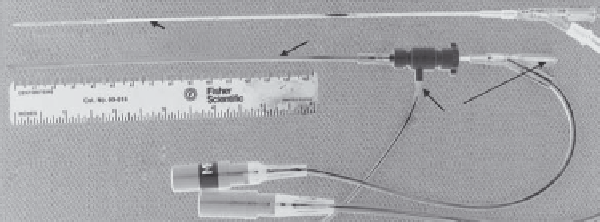
ing hemostasis valve (Figure 11.5a). As mentioned before, the
enhanced radial penetration of ultrasound allows larger appli-
cator separation (2-3 cm) of heating devices, and directional
applicators can be selected to either protect nontargeted tissues
(e.g., bladder, rectum) or preferentially target eccentric tumor
volumes. Examples of a typical patient setup and temperature
distributions achieved are shown in Figure 11.5b for the treat-
ment of prostate cancer using three applicators placed in the
posterior lower portion of the gland; 180° directional applica-
tors are placed and aimed anterior toward the hyperthermia
target volume and away from the rectum, ensuring sparing of
the thermally sensitive rectal wall. A second example is dem-
onstrated in Figure 11.5c, for preferentially targeting a necrotic
area of a cervical tumor. Spatial control and penetration char-
acteristics allow tissue protection and thorough therapeutic
temperatures within the target and protection. This spatial
control and penetration is not possible with other interstitial
hyperthermia modalities such as MW and RF [8].
(a)
13-g catheter
Ultrasound applicator
Water-flow
ports
Quick-connects
RF power
40.8°C
43.6°C
(c)
(b)
41.1°C
41.4°C
Bladder
HTV
CTV
44.4°C
HTV
41.9°C
47.4°C
44.3°C
Rectum
45.3°C
1cm
1cm
Rectum
FIGURE 11.5
Catheter-based or interstitial ultrasound applicator based upon arrays of tubular transducers and clinical implementation for
hyperthermia integrated with HDR: (a) multi-transducer applicator with 180° heating pattern and control of heating profile along length, within
plastic implant catheter; (b) example prostate treatment with three directional heating applicators directing energy away from rectum toward
target zone, with measured therapeutic temperatures; (c) example treatment of cervical cancer, applicators are within periphery and aligned to aim
into the target zone, also shown with measured temperatures.