Biomedical Engineering Reference
In-Depth Information
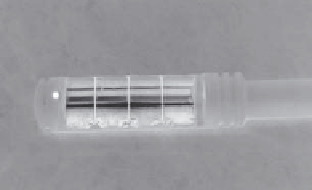
FIGURE 11.1
Transrectal ultrasound applicator for prostate hyperthermia demonstrating multi-sectored transducer segments that give angular
and longitudinal control of heating directed to the prostate, and the water-cooled coupling balloon. (Photos courtesy of Dr. Kullervo Hynynen,
Sunnybrook Health Sciences Centre, and Dr. Mark Hurwitz, Dana-Farber/Brigham & Women's Hospital, Harvard Medical School.)
adjustable for tailoring the heating distribution to fit the intended
target region extending from apex to base. Studies have indicated
that these applicators could therapeutically heat tissues 3-4 cm
deep from the rectal cavity wall, which is sufficient to treat most
prostate glands, while proper cooling of the bolus will maintain
the rectal mucosa at subtherapeutic temperatures. Devices of this
design scheme have been implemented in a phase I feasibility and
toxicity trial [19], which evaluated transrectal ultrasound hyper-
thermia given with concurrent standard external beam irradia-
tion in the treatment of locally advanced adenocarcinoma of the
prostate. Therapeutic temperatures ranging between 40.6°C and
43.2°C were reported for a total of 14 patients. Advanced versions
of this applicator have added four sectors on each tubular section
(Figure 11.1), for 16 channels total, thus adding additional con-
trol of the heating in the angular expanse as well as longitudinal
control [18]. MR compatible versions of this device and control
algorithms for feedback control have been investigated and dem-
onstrated that MR directed hyperthermia with this approach is
feasible [21,22], including demonstration of heat-directed gene
delivery [23] to selected regions of the prostate. Transrectal ultra-
sound hyperthermia applicators demonstrate improved heating
penetration and spatial control of power deposition compared
to capabilities of transrectal microwave techniques, providing an
improved technique to heat more of the gland without complica-
tions to rectal tissue.
Delivering effective and therapeutic hyperthermia to the
whole prostate gland is achievable with this investigational
device, with no increase of rectal toxicity [24,25]. Recent analy-
sis of clinical data by Hurwitz et al. has shown that endorectal
hyperthermia delivered concurrently with external beam radia-
tion yields improvement in survival over other therapies [26].
These applicators are ideally suited for applying conventional
hyperthermia to the whole prostate gland, and may be useful for
radiation or chemotherapy plus heat, and thermal targeted drug
delivery directly to the prostate.
France) [27-29]. These transrectal HIFU systems utilize sharply
focused ultrasound transducers that produce small intense focal
patterns capable of producing selective or well-localized high-
temperature thermal damage within the prostate while avoiding
nontargeted surrounding tissues such as rectum and the neuro-
vascular bundles (NVB).
The Sonoblate system consists of a mechanically scanned
fixed focus HIFU applicator integrated with B-mode imaging
capabilities (Figure 11.2a-c). The imaging component, referred
to as split beam technology [30], yields transverse and longitu-
dinal images for treatment setup and monitoring during treat-
ment. The HIFU applicator consists of a 30 mm long × 22 mm
wide curved transducer operating at 4 MHz with a 25-45 mm
variable focal depth selected
a priori
based upon size and shape
of the prostate. Typically 3-4 s high intensity sonications or
periods of applied power produce ~ 3 × 3 × 10-12 mm
3
coagu-
lated tissue zones per shot, with a 6-12 s delay between shots.
The Ablatherm endorectal applicator (Figure 11.2d-f) is similar,
with a focused therapy transducer operating at 3 MHz with fixed
focus at 40 mm depth that is robotically positioned and coupled
with a 7.5 MHz imaging array. The Ablatherm produces coagu-
lation zones of ~1.7 mm × 19-26 mm adjustable length for each
shot. These short exposures to high temperatures generate lethal
thermal doses that generate a well-defined zone of thermal coag-
ulation and necrosis. The endorectal applicator on each of these
devices is water-cooled within an expandable balloon to ~20°C
to protect the rectum from excess thermal exposure as well as
couple the ultrasound energy and adjust the depth of focus.
The ultrasound imaging is used for accurate anatomical posi-
tioning, tissue targeting, and real-time monitoring during treat-
ment (Figure 11.2c). Based upon an image-based treatment plan,
the power levels and positions of required sonication points and
subsequent thermal lesions are mechanically stepped in time to
thermally coagulate a larger contiguous treatment volume. Each
of these systems utilizes different treatment strategies [29]: the
Sonoblate 500 treats three coronal layers, from anterior to poste-
rior; the Ablatherm targets four to six volume boundaries in axial
slices extending from apex to base. During the treatment session,
US imaging can be used to qualitatively assess the treatment pro-
gression and provide feedback as to generation of a thermal lesion
based upon changes in backscatter or attenuation [13].
The Sonoblate was initially applied to treatment of benign
prostatic hypertrophy (BPH) and later utilized for treating
11.2.2 transrectal HIFU for prostate therapies
11.2.2.1 Ultrasound Guided Systems
There are two commercial systems available for transrectal high
intensity focused ultrasound (HIFU) under ultrasound (US)
guidance for thermal ablation of prostate tissue: the Sonoblate
500 (Misonix, U.S.) and the Ablatherm (EDAP TMS, Lyon,