Biomedical Engineering Reference
In-Depth Information
before the system is first used and periodically thereafter (Gorny
et al. 2006, 2009). Specialized training on the use of both the
MR scanner and the focused ultrasound system is critical for
ensuring proper performance and safe operation. Briefly, the QA
procedures include: (1) checking the water level in the cooling
system reservoir; (2) checking the treatment table docking to the
MR scanner; (3) connecting power to the treatment table (the
water hose also needs to be connected to the treatment table for
breast treatments); (4) testing of the MR coil and MR images; (5)
calibrating the transducer position; (6) ensuring that the images
shown on the treatment workstation are the correct images; (7)
calibrating the effective focal spot in two directions (coronal and
axial or coronal and sagittal) using MR thermometry; and (8)
testing all the safety devices alluded to in Section 10.2.1.1.5.
fibroid treatment, including optimized treatment planning, new
types of spots, and electronically customized transducer aper-
tures to allow precise energy delivery to tissues adjacent to bow-
els or to other acoustic obstacles.
10.2.2.1 Conformal Bone transducer
The conformal bone transducer (with straps and patient support
accessories) is an option integrable into the ExAblate 2100 system.
The transducer is a phased array transducer with 1000 elements
operating at a frequency of 550 kHz. The phase (and amplitude)
to each transmitting element is digitally controlled and monitored
during sonications. Therapeutic bursts of 1000 J to 2000 J of energy
are typically delivered to coagulate the targeted bone tissue inter-
face area using multiple sonications. The conformal transducer
positioning and strapping features enable flexible patient position-
ing and access to any desired treatment site in the human body.
The built-in water circulation system includes active cool-
ing to maintain the skin temperature at about 20°C. The water-
impermeable membrane provides acoustic coupling from the
phased array to the skin. The system monitors and controls the
water temperature, protects against leaks, and prevents bubble
formation. The conformal bone transducer comes with an inte-
grated tracking coil, which allows it to locate the position and
angulations of the transducer while inside the MR bore.
10.2.2 Exablate 2100/Exablate
®
Or
The first MRgFUS treatment of a uterine fibroid was performed
in 2001 using the ExAblate 2000 system. Since then, thousands
of patients have been treated in more than 60 different hospitals
globally. The knowledge accumulated from the clinical use of
this new treatment technology has been implemented by vendors
into software and hardware updates. It has also led to clinical
guidelines for improved clinical outcomes and maintaining of a
high level of safety. The updated ExAblate system was named the
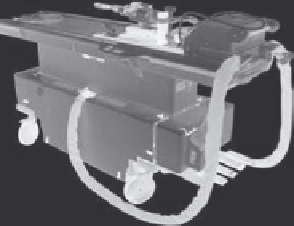
ExAblate®OR (Figure 10.4a). The major advances of the ExAblate
2100 system over the ExAblate 2000 system are: (1) the table
contains additional devices for the integration of both the con-
formal bone transducer (Figure 10.4b) and the endorectal FUS
transducer; (2) the transducer in the water tank can be moved in
the vertical direction; and (3) various improvements for uterine
10.2.2.2 Endorectal FUS transducer
An endorectal FUS transducer is also an available option. It can
be integrated into the modified ExAblate 2100 system for pros-
tate cancer clinical trials. Patients with biopsy proven low risk
prostate cancers were treated using the MRgFUS ExAblate sys-
tem in Italy, Russia, Singapore, and India. Figure 10.5 shows an
(a)
(b)
FIGURE 10.4
(a) The ExAblate 2100 UF V2 system, and (b) the conformal bone transducer. (Courtesy of InsighTec, Inc.)
FIGURE 10.5
The Endorectal FUS transducer. (Courtesy of InsighTec, Inc.)