Biomedical Engineering Reference
In-Depth Information
The perfusion coefficient,
w
bl
, is often provided as a function of
temperature. Inflammation due to mild hyperthermia increases
local blood flow, which is captured by an increase in the perfusion
coefficient from 40-46°C. However, at temperatures above 50°C,
blood coagulation and tissue contraction lead to a rapid decrease
in blood perfusion and eventual thrombosis (Craciunescu 2001,
Tompkins 1994, Rawnsley 1994). This effect is frequently mod-
eled using a Heaviside step or other sigmoidal function for the
perfusion coefficient with respect to temperature.
types of tissues, including water vapor and dehydrated, charred,
or desiccated tissues created during the ablative process. The
result is that microwaves can be applied continuously through-
out the ablation procedure.
The wavelength and penetration depth of microwaves in
tissue is also suitable for a variety of medical applications. At
915 MHz and 2.45 GHz, wave penetration is 2-4 cm in most
tissues, which is commensurate with the majority of tumors tar-
geted by thermal ablation. By contrast, power deposition atten-
uates rapidly away from RF electrodes and optical fibers used
for laser ablation. As a result, microwaves may produce more
active heating than these other energy sources. Active heating
is thought to be more potent against heat loss to blood perfusion
or large vascular heat sinks. Indeed, while RF ablation has been
shown to be relatively ineffective near vessels 3 mm or larger in
diameter, microwaves are effective against vessels at least 3 mm
in diameter and have been shown to induce complete thrombo-
sis of vessels up to 10 mm in diameter (Yu 2008, Lu 2002, Brace
2007a, Wright 2003). Other studies have shown that microwaves
produce larger zones of ablation in liver, kidney, and lung tissues
when compared to RF ablation, even when the applied power
was held constant (Andreano 2010, Brace 2009a, Laeseke 2009).
Other studies have also shown that by using higher powers and
shorter treatment times, microwaves may actually be more effec-
tive in vivo than ex vivo, an effect not noted with other ablation
energy types to date (Hines-Peralta 2006).
Microwave energy is not without drawbacks, however. As
mentioned earlier, the 2-4 cm wavelength penetration of micro-
wave energy in tissue means that heating precision may be sac-
rificed with microwaves. Rapid heating and high temperatures
also need more critical safety evaluation, especially when applied
for long times (several minutes) to a large volume of tissue. This
is especially true from a monitoring standpoint, when rapid
heating might be difficult to capture without real-time imaging.
Microwave energy can also overheat the cabling used to transfer
power from the generator to the applicator. This internal cable
heating must be offset either by limiting energy transmission or
by active cable cooling to prevent unwanted thermal damage to
tissues along the cable length (He 2010). However, even when
cooled, many antenna designs produce elongated ablations,
which may not be desirable for many clinical applications (Tse
2009, Sun 2009, Brace 2007b). Many of these deficiencies may be
overcome by improved system component design and optimized
power application protocols.
9.4.3 Microwaves Compared to Other
Sources of thermal therapy
Microwave energy has unique properties that make it an attrac-
tive choice for thermal ablation. The most-cited advantages for
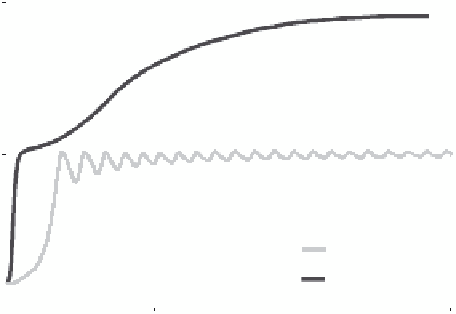
microwave ablation are a faster heating rate (Figure 9.12), greater
volume of active or direct heating, higher temperatures inside
the ablation zone, less susceptibility to ablation-induced changes
such as charring or desiccation, larger zones of ablation, and
improved performance with multiple applicators. These com-
parisons are often drawn with RF ablation, but may also apply
to varying degrees in comparisons to laser sources, ultrasound
sources, or cryoablation. The common thread to these potential
advantages is the fact that microwave heating occurs as a result
of electromagnetic propagation through biological tissues of
all types. Radiofrequency, laser, and ultrasound energy sources
can be significantly hampered by specific types of tissue. For
example, lung and bone tissues are poor electrical conductors at
RF frequencies, which limits the amount of current and, hence,
power that can be deposited during RF ablation (Duck 1990).
Charred and desiccated tissues are also poor conductors of RF
electrical current, and present a barrier to laser light penetration
and ultrasound wave propagation. Consequently, RF, laser, and
ultrasound sources are typically modulated or tempered dur-
ing ablation treatments to avoid generating temperatures over
100°C. On the other hand, microwaves propagate through all
175
150
125
100
75
9.5 Microwave ablation Systems
RF
Microwave
50
The microwave ablation system consists of three main com-
ponents: a microwave power generator, a system for delivering
power to the applicator antenna, and the antenna itself. Of these,
antenna design has been given the most discussion in the existing
literature; however, techniques for distributing power to multiple
antennas and the role of frequency have also been investigated. A
more detailed discussion of each component follows.
25
0
100
200
300
Time (s)
400
500
600
FIGURE 9.12
Temperatures measured 5 mm from a microwave
(MW) and RF applicator during ablation of the renal cortex demon-
strate more rapid and continuous heat generation.