Biomedical Engineering Reference
In-Depth Information
Method in the Time-Domain) is used. Unconditionally stable
ADI-FD (ADI Finite-Differences) schemes have been devised to
allow arbitrarily large stable time steps [142, 181]. Discretization
and staircasing errors in FDTD have been studied in [150, 123],
and various methods have been presented to reduce staircasing
errors [131, 150, 196]. Of particular interest is a conformal cor-
rection scheme that has been applied in the context of hyper-
thermia [123]. It was found that the impact of staircasing in the
context of hyperthermia for a relevant example case accounted
for 1.5°C [122].
Various techniques have been used to speed up thermal simu-
lations. These include hardware acceleration (GPU and CELL
BE chip [33]), parallelization [33], and numerical techniques
such as adaptive mesh refinement [56], region decoupling [122],
narrow-band update schemes for temperature-dependent tissue
parameters and phase transitions [122], and steady-state estima-
tion-based initialization [122].
have been applied range from genetic algorithms [124, 156] to
the generalized Eigenvalue method [91, 92, 124], particle swarm
optimization, interior point optimization [182, 32], constrained
sequential quadratic programming [93, 164], and a modified
Newton Method [132]. The generalized Eigenvalue method is
particularly fast but puts strong constraints on the possible form
of the optimization functional and allows for virtually no addi-
tional constraints (e.g., load balancing between multiple amplifi-
ers/antennas). These disadvantages can be overcome with genetic
algorithm-based methods [124]. PDE constraint interior point
optimization [182, 32] allows the coupled (nonlinear) thermal
simulation/optimization problem to be solved while providing
maximal flexibility with regard to the functional and allowing
for nonlinear thermal models (e.g., thermoregulation). Different
optimization functionals have been used [75, 94]. Some have
tried to minimize the difference to a target distribution; oth-
ers have tried to maximize the ratio of the averaged exposure
in the target region to the averaged exposure of the other areas.
A third approach tries to maximize the exposure of the target
regions while restricting the tolerated exposure of healthy tissue.
A detailed review of many optimization functionals and their
advantages is given in [21].
Approaches that require repeated evaluation of the functional
have attempted to speed up the calculation process by bringing
it into a form that can be largely precomputed (e.g., [122, 125,
185]). Some functionals include the weighting of different tis-
sues with specific sensitivities to heat. [91, 124] perform a first
optimization to identify likely hot spot locations and then repeat
the optimization process with increased weight for these prob-
lematic regions—a process called “hot spot suppression.” When
the contributions of different regions are precomputed, rapid
reoptimization with reweighted regions (e.g., based on patient
feedback such as pain complaints as suggested by [22]) can be
performed, thus allowing treatment planning to make the step
into the treatment room.
Various techniques have been used to speed up the optimiza-
tion process by model reduction, e.g., (1) by grouping points that
react in a similar manner to antenna setting changes [41], (2) by
performing a Karhunen-Loeve transformation and only using
the vectors contributing most to the variance, or (3) by using
Eigenvalue-based optimization to find the best settings for indi-
vidual targets and then combining these settings [11]. Another
approach [93] has been proposed that performs high-resolution
optimization based on low resolution field calculations that have
been interpolated using quasistatic zooming and an associated
temperature interpolation method. Most approaches optimize
only the steady state. However, some consider the transient effects
and even the cool down period [4, 5, 30], concluding that some-
times the ideal heating strategy should not aim at generating
homogeneous heating in the tumor, but rather a higher exposure
of the tumor border, and that the most intense treatment is not
necessarily the best or the shortest one. Inverse methods have been
used [61] to determine the ideal EM potential boundary condition
at the patient circumference for optimal heating. Various publica-
tions [4-6, 90, 100] have studied the possibility of implementing
7.6 Field Optimization
Usually it is only the antenna steering parameters that are opti-
mized, whereas the patient position with respect to the anten-
nas and the water bolus temperature are often fixed based on
guidelines (e.g., [44, 171]) as repeated performance of the EM
and thermal simulations would be required to optimize them.
It is expected that, with the increase in computational power,
the latter could also become standard. As optimization is a large
area, only optimization techniques that have been applied in the
field of HTP will be discussed.
Both SAR [11, 91, 139, 156, 185] and temperature (increase)
[42, 91, 93, 94, 100, 101, 132, 164] based optimization approaches
have been investigated (see Figure 7.5). The EM field for each
antenna, or the temperature increase field for each pair of anten-
nas (see [91]), on which the optimization is based, must usually
be precomputed. However, [42, 100] base their optimization on
temperature distributions in the patient for various antenna
settings measured by MRI thermometry. The techniques that
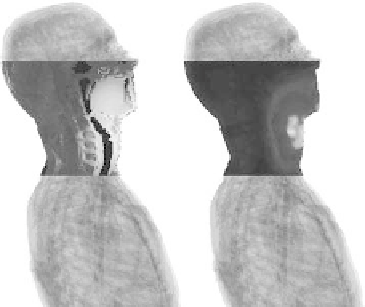
FIGURE 7.5
(Left) Optimized SAR distribution and (right) resulting
temperature increase prediction (see Figure 7.4 for a better view of the
model). Notice how the temperature increase is not perfectly correlated
with the SAR distribution.